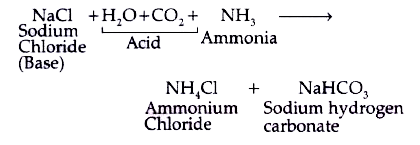Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-ACIDS, BASES AND SALTS-Board Corner (Single Answer Type Questions)
- A compound 'X' of sodium is used as an antacid and it decomposes on st...
Text Solution
|
- A compound 'X' of sodium is used as an antacid and it decomposes on st...
Text Solution
|
- A compound 'X' of sodium is used as an antacid and it decomposes on st...
Text Solution
|
- You are provided with 90 mL of distilled water and 10 mL of concentrat...
Text Solution
|
- You are provided with 90 mL of distilled water and 10 mL of concentrat...
Text Solution
|
- 'Sweet tooth may lead to tooth decay'. Explain why? What is the role o...
Text Solution
|
- In the electrolysis of water, Name the gas collected at anode and ca...
Text Solution
|
- In the electrolysis of water, Why is the volume of gas collected at...
Text Solution
|
- In electrolysis of water, What would happen if dil. H2SO4 is not ad...
Text Solution
|
- pH has a great importance in our daily life. Explain by giving three e...
Text Solution
|
- Identify the compound of calcium which is used for plastering of fract...
Text Solution
|
- A compound which is prepared from gypsum has the property of hardening...
Text Solution
|
- Identify the acid and the base from which sodium chloride is obtained....
Text Solution
|
- Identify the acid and base which form sodium hydrogen carbonate. Write...
Text Solution
|
- While diluting an acid, why is it recommended that the acid should be ...
Text Solution
|
- Dry hydrogen chloride gas does not change the colour of dry litmus pap...
Text Solution
|
- In one of the industrial processes for manufacture of sodium hydroxide...
Text Solution
|
- The pH of a salt used to make tasty and crispy pakoras is 14. Identify...
Text Solution
|