A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise CARBON AND ITS COMPOUNDS (VISUAL CASE - BASED QUESTIONS)|15 VideosMETALS AND NON-METALS
OSWAL PUBLICATION|Exercise Board corner(Long answer type questions)|20 VideosNTSE 2019 - 20
OSWAL PUBLICATION|Exercise (CHEMISTRY) ATOMS AND MOLECULES (STAGE - 2)|1 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION -PERIODIC CLASSIFICATION OF ELEMENTS (VISUAL CASE - BASED QUESTIONS)
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
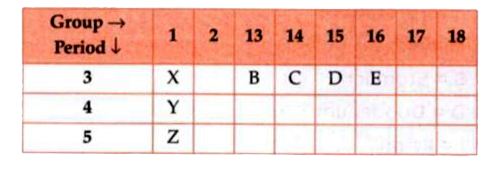
- Which of these elements have smallest atomic size?
Text Solution
|
- Write electronic configuration of element E.
Text Solution
|
- Identify the elements which have similar physical and chemical proper...
Text Solution
|
- The number of period that the modern periodic table has :
Text Solution
|
- An element 'X' belongs to the third period and group 16 of the period...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|