Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-STRUCTURE OF ATOM-LET US ASSESS
- Names of some scientists and their contributions are given shuffled in...
Text Solution
|
- Atomic number of an atom Z=17, Mass Number A= 35. Find the number o...
Text Solution
|
- Atomic number of an atom Z=17, Mass Number A= 35. Write the electro...
Text Solution
|
- Atomic number of an atom Z=17, Mass number 35. Draw the Bohr model ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
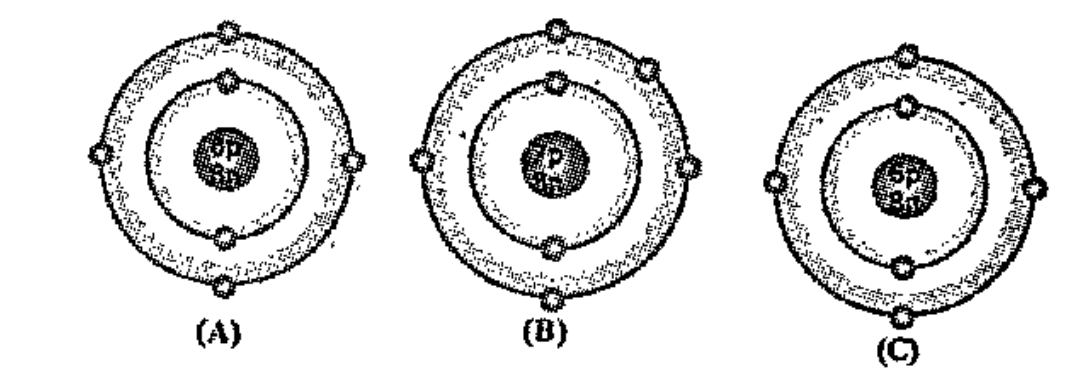
- Bohr models of atoms A, B, C, are given (Symbols are not real). Am...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|