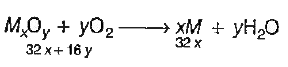A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 4 g of metal oxide MxOy is reduced by H2 and 2.4 g metal is obtained, ...
Text Solution
|
- A metal oxide has the formula Z(2)O(3) . It can be reduced by hydrogen...
Text Solution
|
- On reduction with hydrogen , 3.6 g of an oxide of metal left 3.2 g of ...
Text Solution
|
- On reduction with hydrogen, 3.6 g of an oxide of metal (M) left 3.2 g ...
Text Solution
|
- किसी धातु के 3.6 ग्राम ऑक्साइड का हाइड्रोजन के साथ अपचयन कराने पर 3.2 ...
Text Solution
|
- 0.72 gm of an oxide of a metal M on reduction with H(2) gave 0.64 g of...
Text Solution
|
- 4 ग्राम धातु ऑक्साइड M(x)O(y) को H(2) द्वारा अपचयित किया जाता है और 2....
Text Solution
|
- 4 ग्राम धातु ऑक्साइड M(x)O(y) को H(2) द्वारा अपचयित किया जाता है और 2....
Text Solution
|
- ग्राम धातु को ऑक्सीजन में गर्म करने पर उसका 2.752 ग्राम ऑक्साइड प्राप्...
Text Solution
|