Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 36-CHEMISTRY
- Predict the product:
Text Solution
|
- On reaction with water, a metal carbide gives a colourless gas which b...
Text Solution
|
- What is the increasing order of stability of the three main conformati...
Text Solution
|
- Percentage of cationic vacancies in Fe(0.93) O is:
Text Solution
|
- An aqueous solution of a salt MX2 at certain temperature has a van't H...
Text Solution
|
- How much oxygen is required for the complete combustion of 2.8 kg of e...
Text Solution
|
- At low pressure, the van der Waals equation become:
Text Solution
|
- The second Bohr orbit energy of the hydrogen atom is -328 "kJ mol"^(-1...
Text Solution
|
- Correct statement about chemical adsorption is:-
Text Solution
|
- Heat of neutralisation of H2C2O4 (oxalic acid) is -26 "kcal mol"^(-1)....
Text Solution
|
- Predict the number of unpaired electrons in a square planar d^8 ion.
Text Solution
|
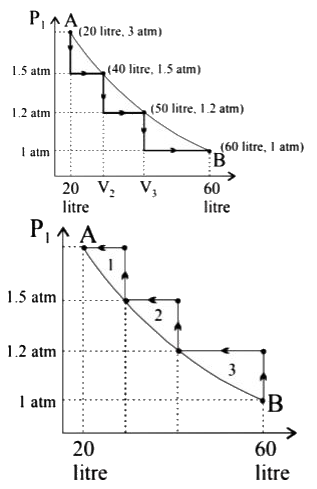
- A certain quantity of an ideal gas is expanded from 20 L to 60 L isoth...
Text Solution
|
- 1026 g of sucrose on hydrolysis gives how many mole(s) of glucose? (At...
Text Solution
|
- How many of the following compounds can be reduced by the Tollen's rea...
Text Solution
|
- CH(3) - underset(Cl)underset(|)CH - CH(2) - CH(3) overset(KOH)underset...
Text Solution
|
- Number of Nitrogen-atoms present in melamine are:
Text Solution
|
- For the non-stoichiometric reaction: A + B to C +D, the following ki...
Text Solution
|
- The standard emf of a Daniel cell is V.
Text Solution
|
- Hemoglobin (Hb) protein transport O2 in blood and each Hb can bind 4O2...
Text Solution
|
- What is the oxidation number of X in an interhalogen compound XY7 ? (X...
Text Solution
|