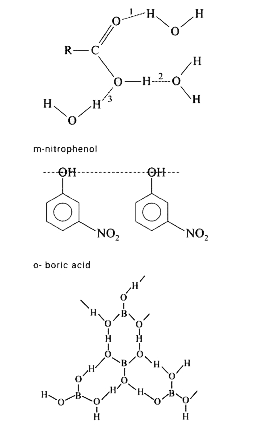
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 43 -CHEMISTRY
- Intermolecular H-bonding can be observed in which of the following ? ...
Text Solution
|
- Which of them do not follow Dobereiner's Triad rule :
Text Solution
|
- Element found in majority in Calamine is
Text Solution
|
- Which of the following statement is correct :
Text Solution
|
- Transition metals are not characterized by :
Text Solution
|
- Correct formula of beryllate ion is :
Text Solution
|
- Reaction between phenyl magnesium bromide and methanol produces
Text Solution
|
- The compound among the following, which undergoes reaction with 50% Na...
Text Solution
|
- Phenyl isocyanides are prepared by which of the following reaction?
Text Solution
|
- The correct IUPAC name of the compound:
Text Solution
|
- The rate constant ki and k(2) for two different reactions are 10 ^(16...
Text Solution
|
- E(1) and E(2) are two half-cells of electrode potential which when c...
Text Solution
|
- 0.1 M CH(3) COOH (pH = 3) is titrated with a 0.05 M NaOH solution. Det...
Text Solution
|
- An element adopts a cubical crystal structure in which only 68% of the...
Text Solution
|
- Which condition is not satisfied by an ideal solution?
Text Solution
|
- 4g of M(2)O (y) oxide was reduced to 2.8 g of the metal in an experim...
Text Solution
|
- Which of the following pair will form H bond?
Text Solution
|
- Select the incorrect statement about photon:
Text Solution
|
- During adsorption the Delta H is - ve and the magnitude of – ve value
Text Solution
|
- Given H(2) (g) + Br(2) (g) to 2 HBr (g), Delta H(1) ^(@) and standar...
Text Solution
|