A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 79-CHEMISTRY
- Among CHCl(3), CH(4) and SF(4) the molecules do not having regular ge...
Text Solution
|
- Which of the following is obtained by Teaching process and can be used...
Text Solution
|
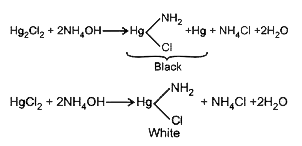
- A metal gives two chlorides 'A' and 'B'.' A' gives a black precipitate...
Text Solution
|
- Among [Ni ( CO ) (4) ] , [Ni (CN) (4) ]^(2-) and [Ni (Cl) (4) ]^(2-):-
Text Solution
|
- Select wrong statement:
Text Solution
|
- Which of the following is not the product of dehydration of
Text Solution
|
- Phenol and benzoic acid can be distinguished by :
Text Solution
|
- Which compound undergoes electrophilic substitution with ease (most r...
Text Solution
|
- Which of the following statements are correct? (i) Sucrose is dext...
Text Solution
|
- Which of the following will not give iodo form test:
Text Solution
|
- Which of the following compounds are meso forms?
Text Solution
|
- Which of the following statements is valid for the above compound?
Text Solution
|
- Reaction by which benzaldehyde can not be prepare :
Text Solution
|
- A solution contains Pb^(+ 2)ion. In order to precipitate Pb^(+2 ) ions...
Text Solution
|
- Antifluorite structure can be obtained from fluorite structure by:
Text Solution
|
- The e/m ratio is maximum for :
Text Solution
|
- In a reaction between A and B, the initial rate of reaction was measur...
Text Solution
|
- If we place 0.5 g Lithium metal in coffee cup calorimeter that already...
Text Solution
|
- Colourless radical among the following: [TiF (6) ] ^(2-), [Co F (6...
Text Solution
|
- IF(7) is an interhalogen compound. The total count of lone pairs in o...
Text Solution
|