A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 42-PHYSICS (SUBJECTIVE NUMERICAL)
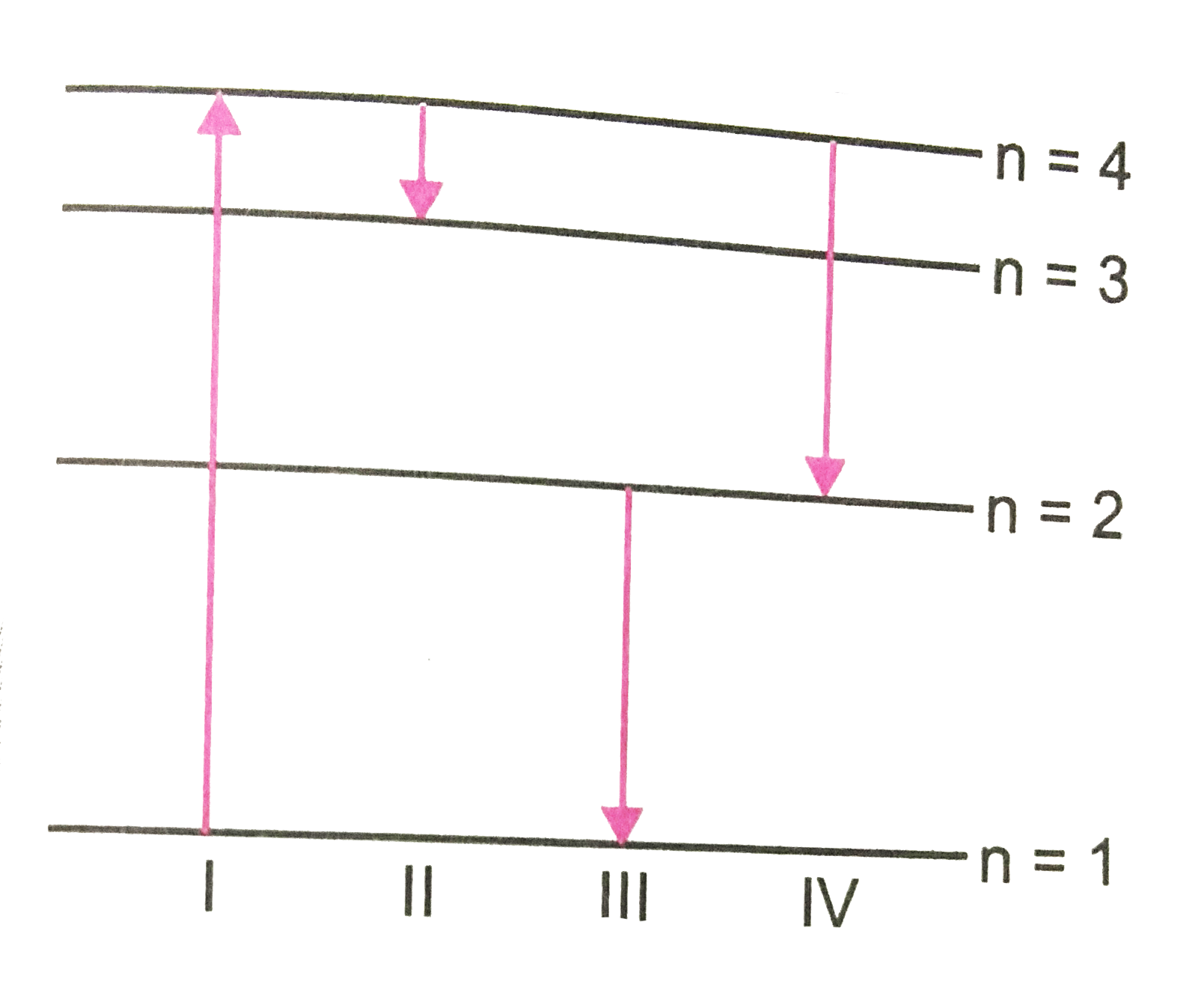
- Shows the energy levels for an electron in a certain atom. Which trans...
Text Solution
|
- The n rows, each containing m cells in series are joined in parallel. ...
Text Solution
|
- The volume charge density of a sphere carrying charge Q and of radius ...
Text Solution
|
- On a planet a ball is dropped from the top of a 100 m high tower. In t...
Text Solution
|
- Two particles A and B have masses 3 kg and 6 kg and velocities 8 m/s a...
Text Solution
|
- The pressure wave, p = 0.01 sin (1000t - 4x] Nm^(-2) corresponds to th...
Text Solution
|
- Two glass plates are placed vertically in liquid with surface tension ...
Text Solution
|
- Consider a string which is suspended from the ceiling. The length of t...
Text Solution
|
- An object when placed in front of a plano-convex lens forms a real ima...
Text Solution
|
- Three identical hollow sphere, each of mass 2 kg and radius 10 cm are ...
Text Solution
|
- A uniform rod of mass M and length l is dropped on horizontal smooth s...
Text Solution
|