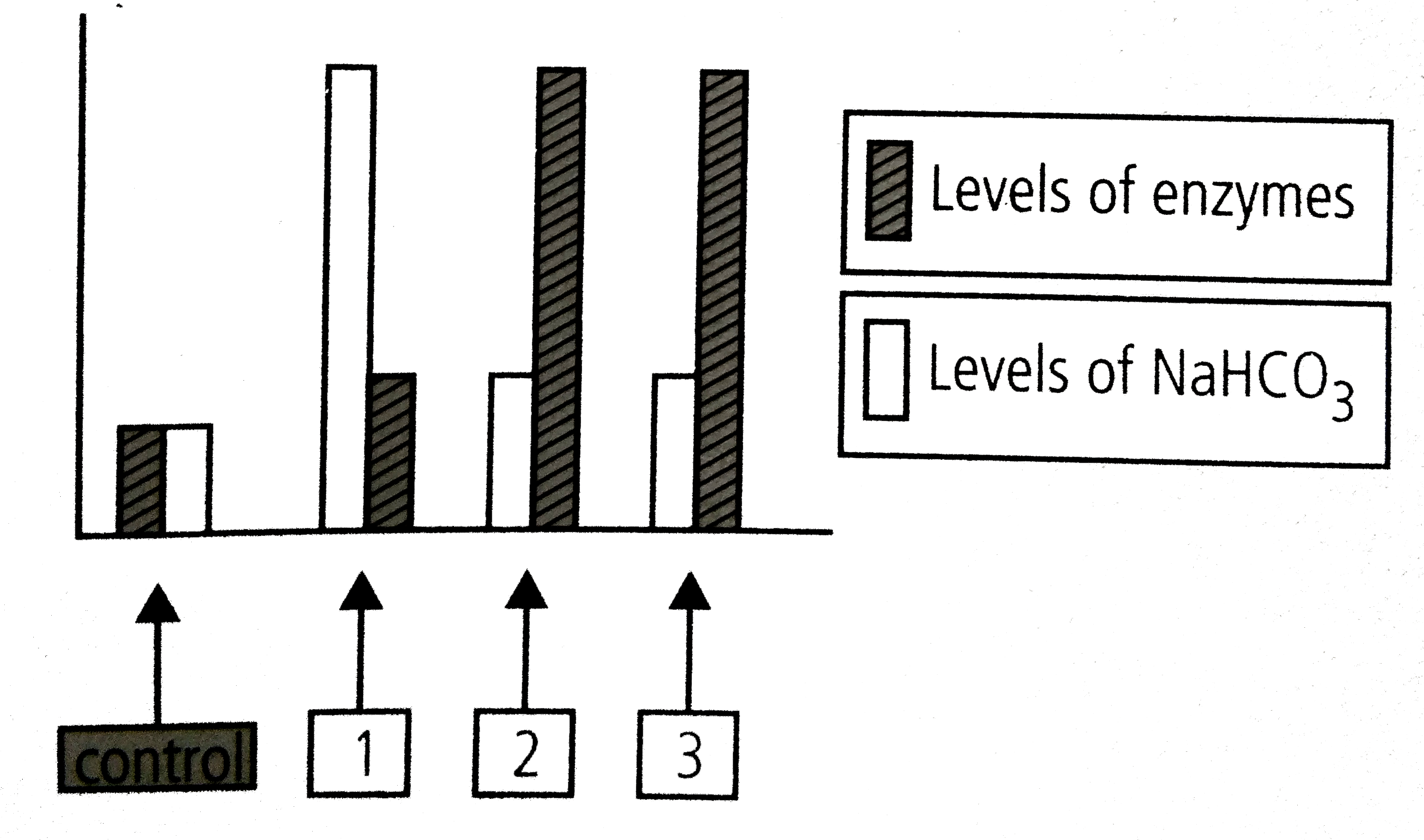A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Effect of some compounds (present in partially digested food) on pancr...
Text Solution
|
- Which of the following component of our food is digested by an enzyme ...
Text Solution
|
- पाचन में अग्न्याशयी रस का क्या महत्त्व है? इसके निष्क्रिय होने पर पाचन...
Text Solution
|
- What is the partially digested food in the mouth ?
Text Solution
|
- Name the partially digested food in the mouth.
Text Solution
|
- Name the partially digested food in stomach.
Text Solution
|
- Partially digested food ...
Text Solution
|
- नीचे दिये गए बार ग्राफ में कुछ यौगिकों (आंशिक रूप से पचित भोजन में उपस...
Text Solution
|
- The partially digested food that comes out of stomach is called
Text Solution
|