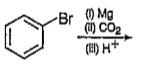
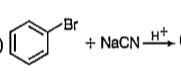
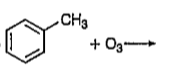
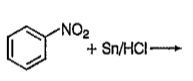A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following reaction shall produce benzoic acid ?
Text Solution
|
- Sulphonation of benzoic acid produces mainly
Text Solution
|
- Which will not produces benzoic acid ?
Text Solution
|
- Which of the following reaction can be used to prepare benzoic acid?
Text Solution
|
- Which of the following will not produce benzoic acid by oxidation with...
Text Solution
|
- In which of the following reaction is benzoic acid the major product ?
Text Solution
|
- Which of the following compounds shall not produce propene by reaction...
Text Solution
|
- Sulphonation of benzoic acid produces mainly
Text Solution
|
- निम्न में से कौन बेन्जोइक अम्ल दुर्बल है?
Text Solution
|