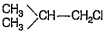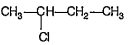A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the reaction with aq. Sodium hydroxide which of the following compo...
Text Solution
|
- Statement-1 : undergoes inversion in DMSO with hydroxide ion Statement...
Text Solution
|
- SN1 + SN2 mixed mechanism is observed in the reaction
Text Solution
|
- Which of the following compound will not undergo amine inversion?
Text Solution
|
- নিম্নলিখিত কোন জৈব ক্লোরিন-ঘটিত যৌগটি SN2 বিক্রিয়ায় সম্পূর্ণ স্টিরিওকে...
Text Solution
|
- Which one of following undergoes reaction with 50% sodium hydroxide so...
Text Solution
|
- In the following pairs of halogen compounds, which would undergo SN2 r...
Text Solution
|
- Which of the following compounds will react with sodium hydroxide?
Text Solution
|
- Which of the following compound will not undergo amine inversion?
Text Solution
|