A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
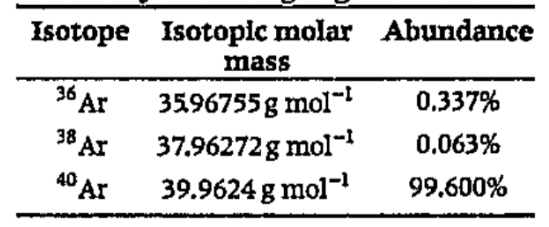
- Use the data given in the following table to calculate the molar mass ...
Text Solution
|
- Use data given in the following table to calculate the molar mass of n...
Text Solution
|
- The average mass of one gold atom in a sample of naturally occuring go...
Text Solution
|
- प्रकृति में उपलब्ध आर्गन द्रव्यमान की गणना के लिए निम्निलिखित त...
Text Solution
|
- प्रकृति में उपलब्ध ऑर्गन के मोलर द्रव्यमान की गणना के लिए निम्नलिखित त...
Text Solution
|
- Use the data given in the following table to calculate the molar mass ...
Text Solution
|
- Calculate the molarity of a solution of ethanol in water in which the ...
Text Solution
|
- Calculate the average atomic mass of naturally occurring magnesium usi...
Text Solution
|
- निम्न सरणी में दिए गए आकड़ो के प्रयोग, प्रकृतिक रूप से पाए जाने वाले आ...
Text Solution
|