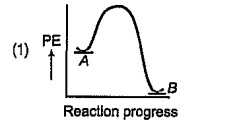
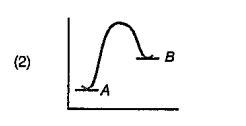
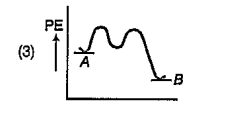
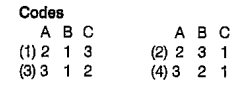
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reaction ArarrB,Ea=10kJ//mol,DeltaH=5kJ//mol . Thus potential en...
Text Solution
|
- For a reaction A rarr B, E(a) = 10 kJ mol^(-1), DeltaH = 5 kJ mol^(-1)...
Text Solution
|
- For a reaction AtoB , E(a) = 10kJ mol^(-1) and DeltaH = 5 kJmol^(-1) ....
Text Solution
|
- The activation energy of exothermic reaction ArarrB is 80 kJ "mol"^(-1...
Text Solution
|
- साधारण रासायनिक अभिक्रिया ArarrB के लिए सक्रियण ऊर्जा, अग्र अभिक्रिया...
Text Solution
|
- For a reaction ArarrB,Ea=10kJ//mol,DeltaH=5kJ//mol . Thus potential en...
Text Solution
|
- The activation energy of the exothermic reaction ArarrB " is "40"kJ."m...
Text Solution
|
- For a reaction, ArarrB , DeltaH = +xkJ .mol^(-1) and activation ener...
Text Solution
|
- For an endothermic reaction, energy of activation is Ea and enthalpy o...
Text Solution
|