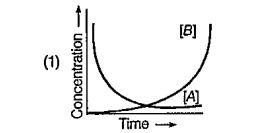
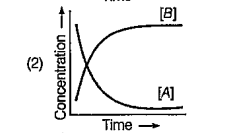
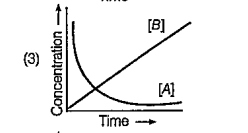
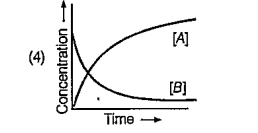
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the reaction A to B. The concentration of both the reactan...
Text Solution
|
- Statement: Concentration of the reactant and product does not change w...
Text Solution
|
- Assertion: For a first order reaction, the concentration of the reacta...
Text Solution
|
- Consider the reaction : A rarr B . The concentration of both reactant ...
Text Solution
|
- Assertion : concentration of the reactant and product does not change ...
Text Solution
|
- Consider the reaction Altimplies B. The concentration of both the reac...
Text Solution
|
- In a first ordr reaction, reactant concentration 'C' varies with time ...
Text Solution
|
- In a 1st order reaction, reactant concentration C varies with times t ...
Text Solution
|
- Assertion. For a first order reaction, the concentration of the reacta...
Text Solution
|