A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
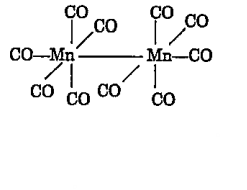
- Decacarbonyl Dimanganese (0) I. is made up of two square pyramidal Mn...
Text Solution
|
- Number of N-Mn-Cl bonds [N-Mn bonds is cis to Mn-Cl bond] in cis [ Mn(...
Text Solution
|
- The IUPAC name for [CO)(5) Mn-Mn (CO)(5)] is
Text Solution
|
- The crystal structure of solid Mn(II) oxide is
Text Solution
|
- Mn(CO)(5) में Mn की ऑक्सीकरण संख्या है
Text Solution
|
- Two Mn(CO)(5) units are joined by bond to form decarbonyl dimanganese...
Text Solution
|
- [Mn2(CO)(10)] is made up of units joined by a Mn-Mn bond.
Text Solution
|
- ठोस Mn (II) ऑक्साइड की क्रिस्टल संरचना होती है
Text Solution
|
- C^(2)mN^(-1) is unit of ...... .
Text Solution
|