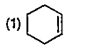
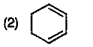
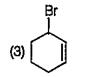
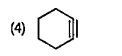
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Heating 1,2 dibromocyclohexane with 2 equivalents of alcoholic KOH yie...
Text Solution
|
- C(6)H(6)CI(6) on treatment with alcoholic KOH yields .
Text Solution
|
- 1,2-dibromopropane is heated with one mole of alcoholic KOH. The react...
Text Solution
|
- Carbylamine test is performed in alcoholic KOH by heating a mixture of...
Text Solution
|
- 4-Formyl benzoic acid on treatment with one equivalent of hydrazine fo...
Text Solution
|
- Phenol on heating with alcoholic with KOH and chloroform undergoes :
Text Solution
|
- A mixture of ethyl amine and alcoholic KOH on heating gives
Text Solution
|
- Which of the following amines yields foul smelling product with halofr...
Text Solution
|
- Ethyl alcohol on heating with HI yield
Text Solution
|