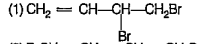A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When 1,3 butadiene reacts with one equivalent of Br2 in a polar solven...
Text Solution
|
- What is the product of the reaction of 1,3-butadiene with Br(2)?
Text Solution
|
- In which of the following reaction 1,3-butadiene will be obtained as a...
Text Solution
|
- Which product is obtained as a major product when one mol of HBr is a...
Text Solution
|
- 1,3-butadiene reacts with ethylene to form
Text Solution
|
- 1,3-Butadiene when treated with Br(2) gives
Text Solution
|
- When trans-butene is reacted with Br2 then product formed is -
Text Solution
|
- A compound A (C8H8O2) react with Br2//FeBr3 and gives only one kind of...
Text Solution
|
- Anisole reacts with Br2 in the presence of CS2 as solvent to give
Text Solution
|