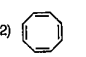
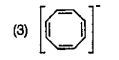
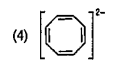
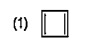To determine which compound among the given options is aromatic, we need to apply the criteria for aromaticity. A compound is considered aromatic if it satisfies the following conditions:
1. It must be cyclic.
2. It must be planar (flat).
3. It must have a continuous ring of p-orbitals (conjugation).
4. It must follow Hückel's rule, which states that it must have \(4n + 2\) π electrons, where \(n\) is a non-negative integer (0, 1, 2, ...).
Let's analyze the options step by step:
### Step 1: Identify the compounds
Assuming we have a list of compounds (for example, benzene, cyclobutadiene, etc.), we will analyze each one.
### Step 2: Check for cyclic structure
For each compound, check if it is cyclic. If it is not cyclic, it cannot be aromatic.
### Step 3: Check for planarity
Determine if the compound can be planar. If the compound cannot adopt a planar structure, it cannot be aromatic.
### Step 4: Count the π electrons
Identify the π electrons in the compound. This can be done by looking at the double bonds and lone pairs that can participate in π bonding.
### Step 5: Apply Hückel's rule
Using the count of π electrons, check if the number of π electrons fits the \(4n + 2\) rule. If it does, the compound is aromatic. If it fits \(4n\), it is anti-aromatic, and if it does not fit either, it is non-aromatic.
### Example Analysis:
- **Benzene (C6H6)**:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 6 (from 3 double bonds)
- Hückel's rule: \(n = 1\) (since \(4(1) + 2 = 6\)), so benzene is aromatic.
- **Cyclobutadiene (C4H4)**:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 4 (from 2 double bonds)
- Hückel's rule: \(n = 1\) (since \(4(1) = 4\)), so cyclobutadiene is anti-aromatic.
### Conclusion:
After analyzing all the compounds, we can conclude which one is aromatic based on the above criteria.