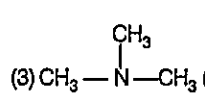
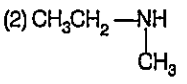A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which among the following has the highest boiling point?
Text Solution
|
- Among the hydrides of group 15 elements which has highest boiling poin...
Text Solution
|
- Among the following , the compound which has highest boiling point is:
Text Solution
|
- Which among the following has highest boiling point?
Text Solution
|
- Among the halogen derivatives of ethane, the one which has the highest...
Text Solution
|
- Which among the following has highest boiling point ?
Text Solution
|
- Which of the following compound has highest boiling point ?
Text Solution
|
- The component of the air among the following which has the highest boi...
Text Solution
|
- Among the following the compound which has highest Boiling point is ?
Text Solution
|