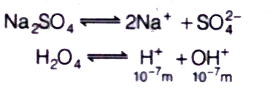A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A solution of sodium sulphate in water is electrolysed using inert ele...
Text Solution
|
- A solution of sodium sulphate was electrolyzed using some inert electr...
Text Solution
|
- If aqueous solutions of AgNO3 is electrolysed using inert electrode th...
Text Solution
|
- If aq. CuCl(2) solution is electrolysed by using Cu electrodes then pr...
Text Solution
|
- An aqueous solution of Na2SO4 is electrolysed using Pt electrodes. The...
Text Solution
|
- An aqueous solution of sodium sulphate is electrolysed using inert ele...
Text Solution
|
- Brine is electrolysed by using inert electrodes. The reaction at anode...
Text Solution
|
- A solution of Na2SO4 in water is electrolysed using inert electrodes. ...
Text Solution
|
- अक्रिय इलेक्ट्रॉडों का प्रयोग करते हुए जल में सोडियम सल्फेट के विलयन क...
Text Solution
|