A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER (SELF-ASSESSMENT )-9-MULTIPLE CHOICE QUESTIONS
- Pleiotropy is the effect of X = gene(s) on Y = ……………….. phenotypic exp...
Text Solution
|
- Which among these are coded by only one codon ?
Text Solution
|
- Which among these are roles played by ribosomes:
Text Solution
|
- Capping II involves the addition of ................ . to the 5'-end o...
Text Solution
|
- The only spot in a seed where the integuments are absent around ovule ...
Text Solution
|
- Which among these characteristics is not required for a substance to b...
Text Solution
|
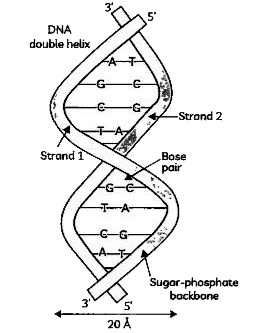
- The given structure was proposed with the help of X-ray diffraction da...
Text Solution
|
- A case dispfaying a pedigree across a family: The trait included ...
Text Solution
|
- The cause of chromosomal disorders is absence or excess of abnormal ar...
Text Solution
|
- In sickle cell anaemia glutamic acid is replaced by valine. Which one ...
Text Solution
|
- Removal of anthers from the flower bud before the anther dehisces in c...
Text Solution
|
- Under which of these conditions there is no need for emasculation?
Text Solution
|
- Pollen tube enters the ovule through ………………………….. .
Text Solution
|
- Emasculated flowers are bagged in order to:
Text Solution
|
- Artificial hybridisation involves:
Text Solution
|
- Bagging is mostly done using-
Text Solution
|
- The given structure was called by another name before the postulation ...
Text Solution
|
- The given procedure is illegal used in many parts of the world to dete...
Text Solution
|
- Name the procedure that can be adopted in an individual having the fol...
Text Solution
|
- Observe the given figiure. lntine is made up of.
Text Solution
|