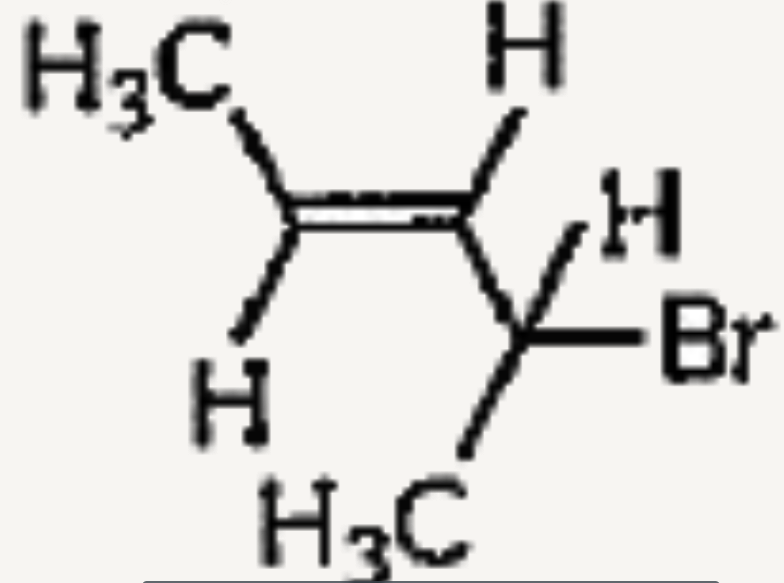
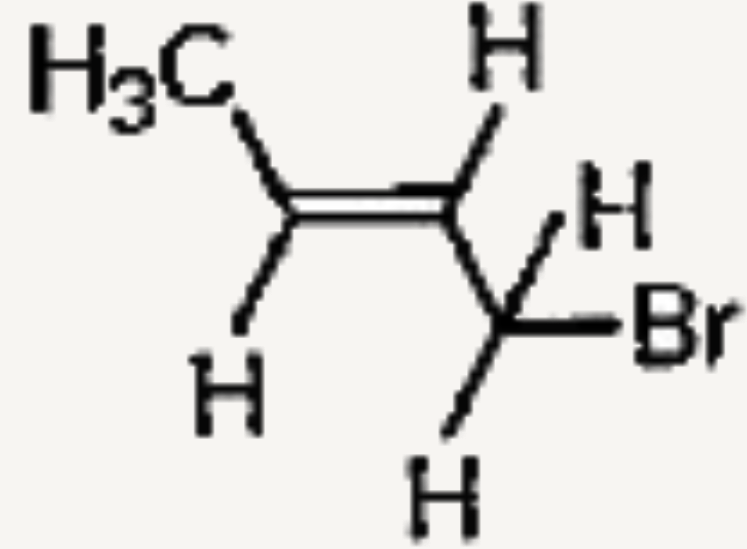
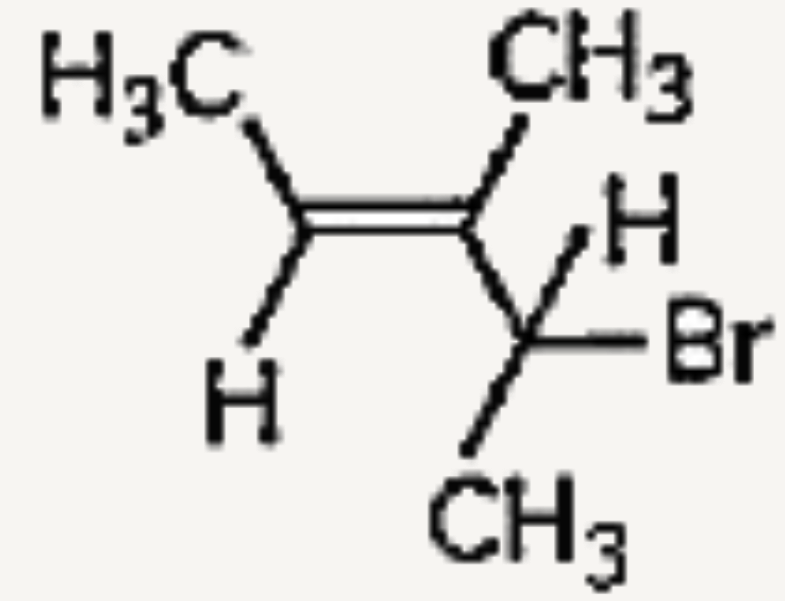
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 06-SECTION-B
- Which one the following alcohol, gives alkenes most readily on acid-ca...
Text Solution
|
- Which of the following. has the highest p pi -p pi bonding tendency?
Text Solution
|
- By heating sodium in the atmosphere of chlorine, sodium chloride is ob...
Text Solution
|
- The incorrect reaction is:
Text Solution
|
- Which is the second most electronegative element in the periodic table...
Text Solution
|
- An azeotropic binary liquid mixture has boiling point lower than eithe...
Text Solution
|
- Which of the following is/are correct about sucrose and maltose:
Text Solution
|
- The increasing order of nucleophilicity is:
Text Solution
|
- In a compound, atoms of element X occupy 2/3rd of tetrahedral voids an...
Text Solution
|
- Ionisation enthalpy of group 15 elements is greater than that of group...
Text Solution
|
- CH3 CH= CH2 to CH3 CH2 CH2 OH . Which reagent can be used for above co...
Text Solution
|
- Name the compound formed cumene undergoes oxidation.
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- What is the IUPAC name of the compound? H3 C - underset(Cl) underset...
Text Solution
|
- The correct structure of 1-bromo-2-methylbut-2ene is:
Text Solution
|
- Assertion (A): SF6 is a well known compound while SH6 does not exist....
Text Solution
|
- Assertion (A): In nucleophilic substitution reactions the presence of ...
Text Solution
|
- Assertion (A): When scuba divers come towards surface, their capillari...
Text Solution
|
- Assertion (A): He and Ne do not form compounds with fluorine. Reason...
Text Solution
|
- Assertion (A): Polar solute dissolve in polar solvents and non-polar s...
Text Solution
|