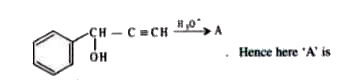A
B
C
D
Text Solution
Verified by Experts
Recommended Questions
- The hydration of alkenes in the presence of acids proceed via carbocat...
Text Solution
|
- Assertion : CH(3)-C-=C-CH(3) is more reactive for electrophilic additi...
Text Solution
|
- Identify the incorrect statement // statement : (i) Alkynes are more r...
Text Solution
|
- Assertion (A): Electrophilic addition addition of Br(2) to alkyne proc...
Text Solution
|
- S-I: Alkynes are more reactive than alkene towards catalytic hydrogena...
Text Solution
|
- Statement-I : Alkyness are more reactive than alkenes towards electrop...
Text Solution
|
- The conjugated dienes are more reactive than alkenes which in turn are...
Text Solution
|
- Why Alkene is more reactivity than Alkyne for Electrophilic Addiition ...
Text Solution
|
- Alkenes and alkynes are unsaturated hydrocarbons and undergo electroph...
Text Solution
|
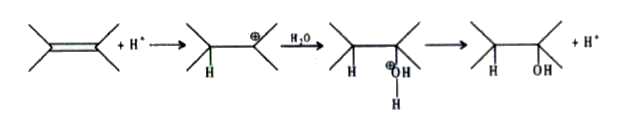 and that carbocation is formed which is most stable, It is observed that alkenes are more reactive than alkynes towards any electrophile. This reaction, i.e., the electrophilic addition reaction of alkenes and alkynes with symmetrical addendum This shows that all reactions of alkenes and alkynes for electrophilic addition reactions may either take place via intermediates of transition state.
and that carbocation is formed which is most stable, It is observed that alkenes are more reactive than alkynes towards any electrophile. This reaction, i.e., the electrophilic addition reaction of alkenes and alkynes with symmetrical addendum This shows that all reactions of alkenes and alkynes for electrophilic addition reactions may either take place via intermediates of transition state.