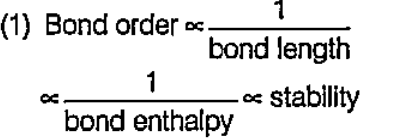
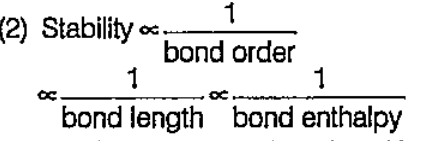
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Recommended Questions
- Which of the following is correct?
Text Solution
|
- Which of the following statements is/are correct about the following i...
Text Solution
|
- Which of the following is/are correct
Text Solution
|
- गेटो के निम्न संयुग्मन के लिए कौन-सी सत्यता सारणी सही है -
Text Solution
|
- Which of the following order is/are correct?
Text Solution
|
- Which of the following are correct for redioactivity ?
Text Solution
|
- Multiple correct postmodern Which of the following statements is corre...
Text Solution
|
- নীচের কোন বক্তব্যটি সঠিক
Text Solution
|
- निम्न में से कौन-सा कथन सही नहीं है?
Text Solution
|