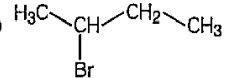
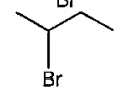
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct way (s) of representing 2 – bromobutane is /are
Text Solution
|
- Convert : a. 1-Bromobutane to 2-bromobutane b. 1-Bromobutane to 1...
Text Solution
|
- If a mixture of 2-bromobutane has enantiomeric excess of 50% of (+)-b...
Text Solution
|
- The back side attack on -- bromobutan by methoxide (CH(3)O^(-)) give...
Text Solution
|
- The reaction of (S)-2 bromobutane with OH^(-) to produce (R )-butane-2...
Text Solution
|
- The back side attack on -- bromobutan by methoxide (CH(3)O^(-)) give...
Text Solution
|
- क्या निम्नलिखित IUPAC नाम सही है ? यदि नहीं तो सही नाम लिखिए । 3-Iod...
Text Solution
|
- Write the mechanism of dehydrohalogenation of 2-bromobutane .
Text Solution
|
- The reaction of (S) - 2 - bromobutane with OH^- to produce (R) - butan...
Text Solution
|