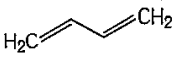
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Recommended Questions
- Which of the following represents the given mode of hybridisation sp^2...
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- Which of the following represents the given mole of hybridization sp^(...
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- Which of the following molecules represents the order of hybridization...
Text Solution
|
- Which of the following represents the given sequence of hybridisation ...
Text Solution
|
- Which of the following represents the given mode of hybridisation sp^...
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|