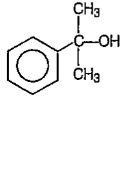
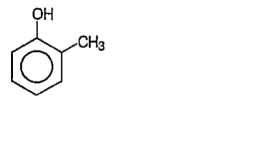
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Recommended Questions
- Choose an allylic alcohol form the given options.
Text Solution
|
- Which of the following is a tertiary allylic alcohol?
Text Solution
|
- Acrolein can be converted into allyl alcohol by
Text Solution
|
- Addition of HOCl to allyl alcohol gives
Text Solution
|
- Identify the allylic alcohols in the above examples.
Text Solution
|
- In allylic alcohol - OH group is attached to
Text Solution
|
- Which of the following is allylic alcohol
Text Solution
|
- IUPAC name of allyl alcohol is
Text Solution
|
- प्रत्येक का एक उदाहरण दीजिये। Allylic alcohol
Text Solution
|