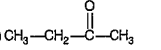A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The product obtained via oxymercuration(HgSO4+H2SO4) Of but-1-yne woul...
Text Solution
|
- The product (s) via - oxymercuration (HgSO(4)+H(2)SO(4)) of 1- butyne ...
Text Solution
|
- The product (s) obtained by the oxymercuration (HgSO4+aq.H2SO4) of but...
Text Solution
|
- When pent-2-yne is treated with dilute H2SO4 and HgSO4 the product for...
Text Solution
|
- The product of oxymercuration of but-1-yne with HgSO(4) and H(2)SO(4) ...
Text Solution
|
- The product(s) obtained via oxymercuration (HgSO(4) + H(2)SO(4)) of 1-...
Text Solution
|
- The product(s) via-oxymercuration (HgSO4+H2SO4) of 1-Butyne would be :
Text Solution
|
- The product(s) obtained via oxymercuration (HgSO(4) + H(2)SO(4)) of 1-...
Text Solution
|
- The product(s) obtained via oxymercuration (HgSO(4) + H(2)SO(4)) of 1-...
Text Solution
|