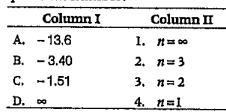
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For an electron in hydrogen atom match values of total energy and its ...
Text Solution
|
- In the Bohr model of the hydrogen atom, the ratio of the kinetic energ...
Text Solution
|
- In the Bohr's model of hydrogen atom, the ratio of the kinetic energy ...
Text Solution
|
- The value of the total energy of an electron in the hydrogen atom is g...
Text Solution
|
- In the Bohr of the hydrogen atom, what is the ratio of the kinetic en...
Text Solution
|
- The total energy of an electron for any particular energy level in hyd...
Text Solution
|
- In the Bohr model of hydrogen atom the ratio of the kinetic energy to ...
Text Solution
|
- हाइड्रोजन परमाणु के बोर प्रतिरूप में इलेक्ट्रॉन की क्वान्टम स्तर में ग...
Text Solution
|
- হাইড্রোজেন পরমাণুর বোর প্রতিরূপ অনুসারে, n কোয়ান্টাম স্তরের ইলেকট্রনে...
Text Solution
|