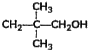
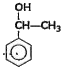
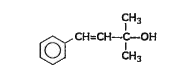
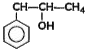A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
EDUCART PUBLICATION-SAMPLE PAPER 04-SECTION B
- What is the structure of BrF5 :
Text Solution
|
- Which is not a reducing sugar?
Text Solution
|
- Which among the flowing compound(s) is/ are primary alcohol?
Text Solution
|
- In which compound oxygen does not show - 2 oxidation state:
Text Solution
|
- Structure of a mixed oxide is cubic closed - packed (ccp) .The cubic u...
Text Solution
|
- Among the given compounds which of the following is vinylic halides:
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following will have lowest vapour pressure?
Text Solution
|
- Which of the following reaction is not the evidence for presence aldeh...
Text Solution
|
- What is the IUPAC name of the compound:
Text Solution
|
- The value of a, b and c values in orthorhombic crystal are 4.2 Å, 8.6 ...
Text Solution
|
- The property of halogens which is not correctly matched is
Text Solution
|
- Which of the following reactions would give the best yield of t-butyl ...
Text Solution
|
- What will the major product of the following : C2H5ONa + CH3-underse...
Text Solution
|
- Which of the following statement is correct about dinitrogen?
Text Solution
|
- Identify X in the following reaction:
Text Solution
|
- Calculate the number of isomeric halopropanes produced, when propane g...
Text Solution
|
- Assertion (A): Nitrogen has diamagnetic in nature. Reason (R): Nit...
Text Solution
|
- Assertion: It is difficult to replace chlorine by -OH in chlorobenzene...
Text Solution
|
- Assertion (R): At low concentration equimolar solutions of non electro...
Text Solution
|