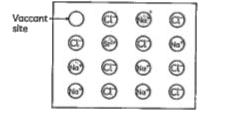
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 05-SECTION-C
- Match the following Which of the following is best matched :
Text Solution
|
- Which of the following analogies is correct:
Text Solution
|
- Complete the following analogy: Two different electron donor site (...
Text Solution
|
- Impurity Defects arises when foreign atoms are present at the lattice ...
Text Solution
|
- Impurity Defects arises when foreign atoms are present at the lattice ...
Text Solution
|
- Impurity Defects arises when foreign atoms are present at the lattice ...
Text Solution
|