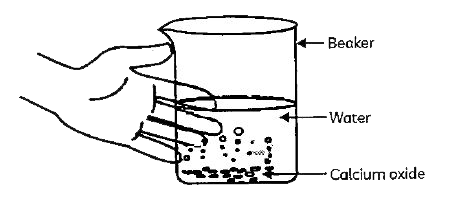
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 2 -SECTION -C
- Identify the type of reaction in the following experiment: (I) Co...
Text Solution
|
- Case 1 : Apoorna took a lime solution and passed a gas 'X' through it ...
Text Solution
|
- Case 1 : Apoorna took a lime solution and passed a gas 'X' through it ...
Text Solution
|
- Case 1 : Apoorna took a lime solution and passed a gas 'X' through it ...
Text Solution
|
- Case 1 : Apoorna took a lime solution and passed a gas 'X' through it ...
Text Solution
|