Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Show that the results given below taken together illustrate a law of c...
Text Solution
|
- 1.375 g of cupric oxide was reduced by heating in a current of hydroge...
Text Solution
|
- 112 mL of hydrogen combines with 56 mL of oxygen of form water. When 2...
Text Solution
|
- What weight of hydrogen at STP could be contained in a vessel that hol...
Text Solution
|
- In the reaction, Hydrogen(g)+ Oxygen (g) rarr watervapour, the ratio o...
Text Solution
|
- What weight of hydrogen at STP couble be contained in a vessel that ho...
Text Solution
|
- 2,75 g of cupric oxide was reduced by heating in a current of hydrogen...
Text Solution
|
- Show that the following results illustrate the law of reciprocal propo...
Text Solution
|
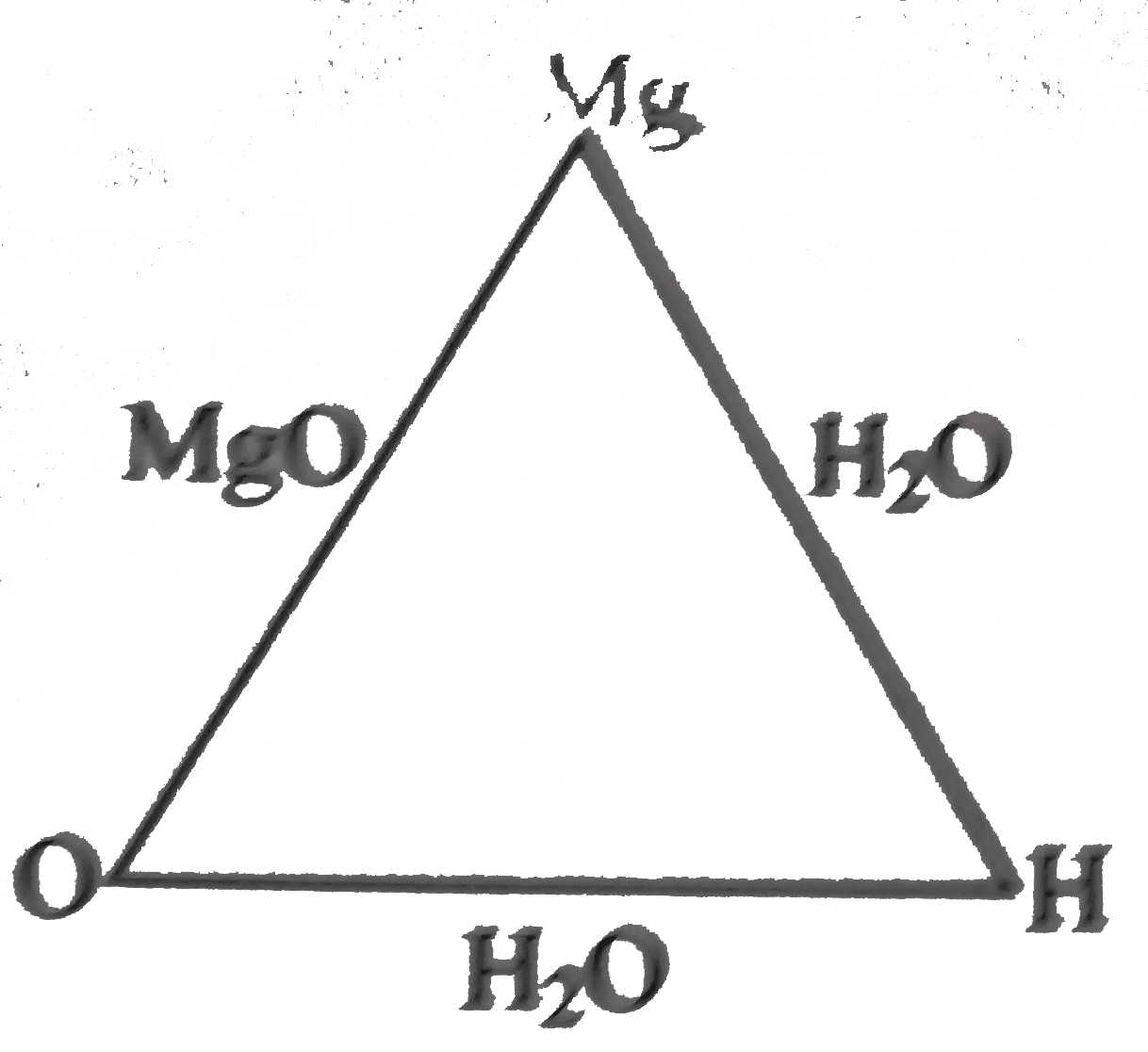
- 28 g of magnesium oxide is produced by the combination of 12 g of magn...
Text Solution
|