Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
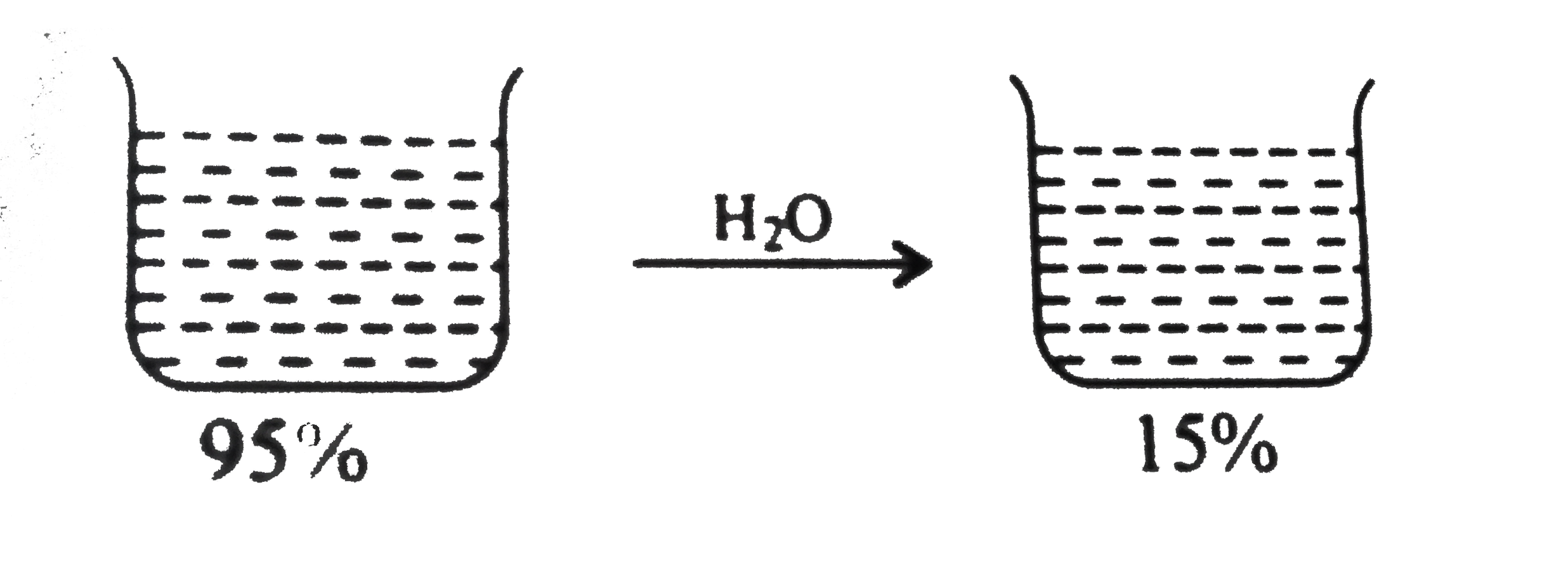
- What volume of 95% H(2) SO(4) by weight (d = 1.85 g mL^(-1)) and what ...
Text Solution
|
- A piece of Al wieghing 2.7 g is titrated with 75.0 mL of H(2) SO(4) (s...
Text Solution
|
- If 100 mL "of" 1 M H(2) SO(4) solution is mixed with 100 mL of 98% (W/...
Text Solution
|
- If 100 mL of H(2) SO(4) and 100 mL of H(2)O are mixed, the mass percen...
Text Solution
|
- 100 mL of 0.1 M solution of H(2)SO(4) is used to prepare 0.05N solutio...
Text Solution
|
- What would be the molality of a solution obtained by mixing equal volu...
Text Solution
|
- Find the molality of H(2)SO(4) solution whose specific gravity is 1.9...
Text Solution
|
- A solution is made by dissolving 49 g of H(2)SO(4) in 250 mL of water....
Text Solution
|
- What volume of 96% H(2)SO(4) solution (density 1.83 g/mL) is required ...
Text Solution
|