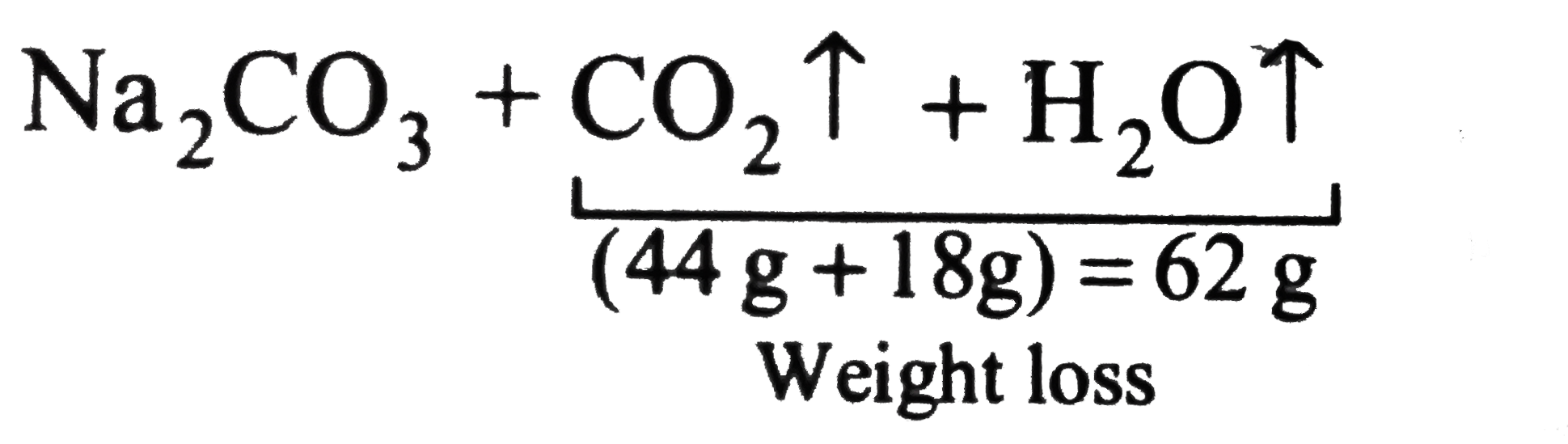Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A 2.0 g of mixture of Na(2)CO(3) and NaHCO(3) loses 0.248 g when heate...
Text Solution
|
- 10 g mixture of NaHCO(3) and Na(2)CO(3) has 1.68 g NaHCO(3) . It is he...
Text Solution
|
- A sample contains a mixtrure of NaHCO(3) and Na(2)CO(3). HCl is adde...
Text Solution
|
- 25.2 gm of a mixture of NaHCO(3) and Na(2)CO(3) is heated strongly, 0....
Text Solution
|
- The 156ml of 0.1 M HCl required to react completely with a mixture of ...
Text Solution
|
- What will be the % loss in mass, if an equimolar mixture of NaHCO(3) a...
Text Solution
|
- A sample contains a mixture of NaHCO(3) and Na(2)CO(3).HCl is added to...
Text Solution
|
- निर्जल Na(2)CO(3) तथा NaHCO(3) के एक मिश्रण के द्रव्यमान में 0.372 ग्र...
Text Solution
|
- A 2.0g mixture of Na(2)CO(3) and NaHCO(3) suffered a loss of 0.12g on ...
Text Solution
|