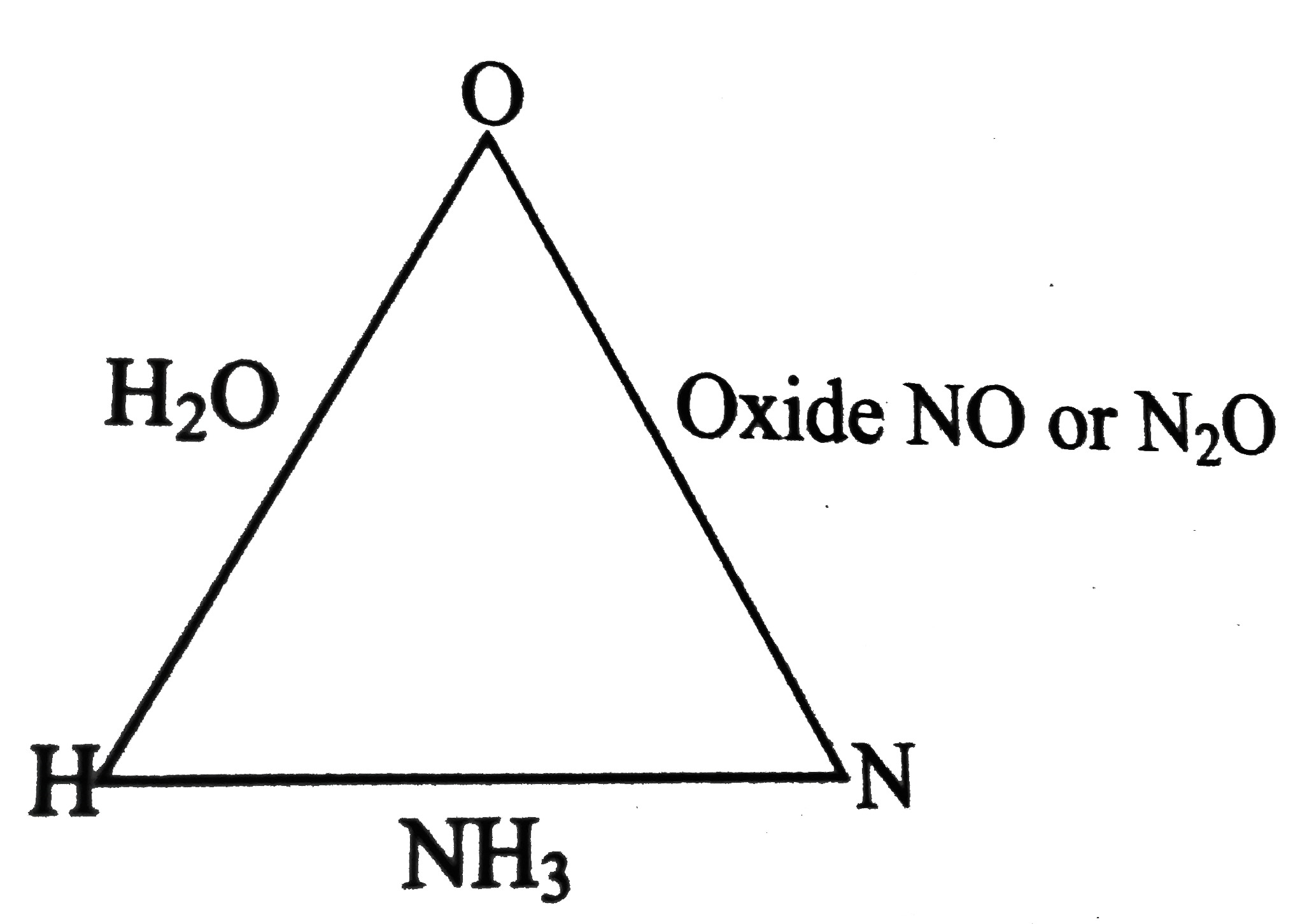
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1 g of oxygen combines with 0.1260 g of hydrogen to form H(2)O. 1 g o...
Text Solution
|
- Nitrogen combines with oxygen to form nitric oxide, N(2(g))+O(2(g))hAr...
Text Solution
|
- In compound A, 1.00 g nitrogen units with 0.57 g oxygen. In compound B...
Text Solution
|
- Hydrogen combines with oxygen with oxygen to form H(2)O in which 16 g ...
Text Solution
|
- 2 g hydrogen combine with 16 g of oxygen to from water and with 6 g of...
Text Solution
|
- 6 g of carbon combines with 32 g of sulphur to form CS(2) . 12 g of C ...
Text Solution
|
- It was found that 16.5 g of a metal combine with oxygen to form 35.6 g...
Text Solution
|
- It is found that 16.5 g of metal combine with oxygen to form 35.60 g o...
Text Solution
|
- In compound A, 1.00 g nitrogen units with 0.57 g oxygen. In compound B...
Text Solution
|