Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
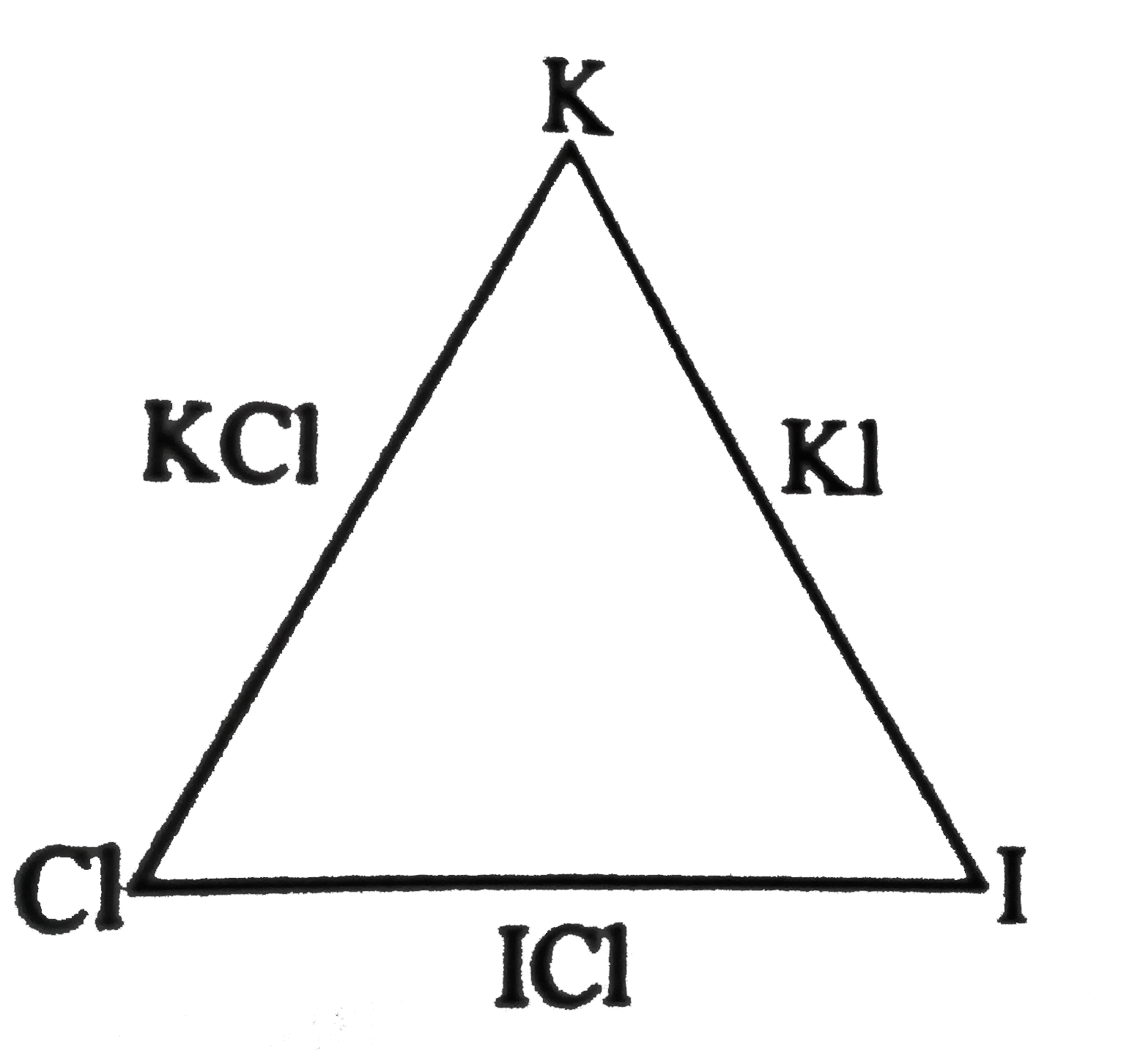
- KCL contains 52 % of potassium, Kl contains 23.6% of potassium, and IC...
Text Solution
|
- Aluminium oxide contains 52.9% aluminium and carbon dioxide contains 2...
Text Solution
|
- A metal oxide contains 53% metal and carbon dioxide contains 27% carbo...
Text Solution
|
- Phosphorus trichloride (PCl(3)) contains 22.57 % of phosphorus, phosph...
Text Solution
|
- Hydrogen sulphide (H(2)S) contains 94.11 % sulphur. Sulphur dioxide (S...
Text Solution
|
- Hydrogen peroxide and water contain 5.93 % and 11.2 % of hydrogen resp...
Text Solution
|
- Verify the law of reciprocal proportions from the following data : KCl...
Text Solution
|
- Ammonia contains 82.32% of nitrogen and 17.65 % of hydrogen . Water co...
Text Solution
|
- Carbon dioxide contains 27.27% of carbon, carbon disulphide contains 1...
Text Solution
|