A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
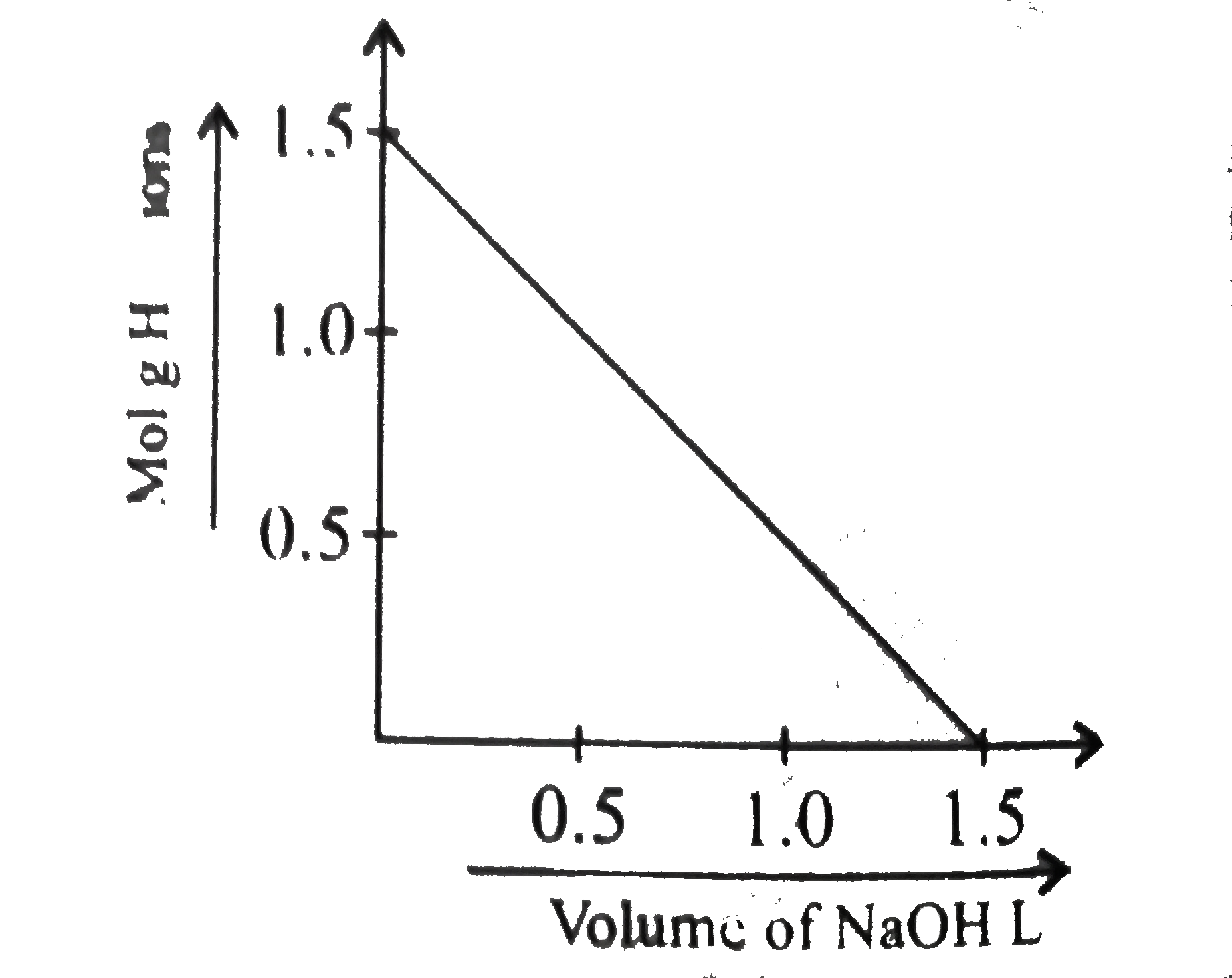
- To 1 L of 1.0 M impure H(2)SO(4) sample, 1.0 M NaOH solution was added...
Text Solution
|
- To 1 L of 1.0 M impure H(2)SO(4) sample, 1.0 M NaOH solution was added...
Text Solution
|
- 100ml of 0.2 M H(2)SO(4) is added to 100 ml of 0.2 M NaOH. The resulti...
Text Solution
|
- Comprehension # 7 Oleum is considered as a solution of SO(3) in H(2)SO...
Text Solution
|
- 1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H(...
Text Solution
|
- 0.7g of (NH(4))(2)SO(4) sample was boiled with 100mL of 0.2 N NaOH sol...
Text Solution
|
- how should a student prepare 100mL of a 1.0 M H(2)SO(4) solution from ...
Text Solution
|
- 0.80g of impure (NH(4))(2) SO(4) was boiled with 100mL of a 0.2N NaOH ...
Text Solution
|
- 1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H(...
Text Solution
|