a. `HCN`: The evaluation cannot be made directly in some cases, e.g., `HCN`, by using rules proposed earlier as we have no rule for the evaluation of oxidation number of both `N` and `C`. In all such cases, evaluation of oxidation number should be made using indirect concept or using fundamentals by which following rules have been formed:
i. Each covalently bond contribures one unit of oxidation number.
ii. Covalently bonded atoms with less electronegativity acquire positive oxidation number whereas other atoms with more electronegativity acquire negative oxidation number.
iii.In case of coordinate bond, give `+2` value for oxidation number to the atom from which coordinate bond is directed to a more electronegative atom.
If coordinate bond is directed from a more electronegative to a less electronegative atom, then neglect the contribution of coordinate bond of both atoms in which coordinate bond exists.
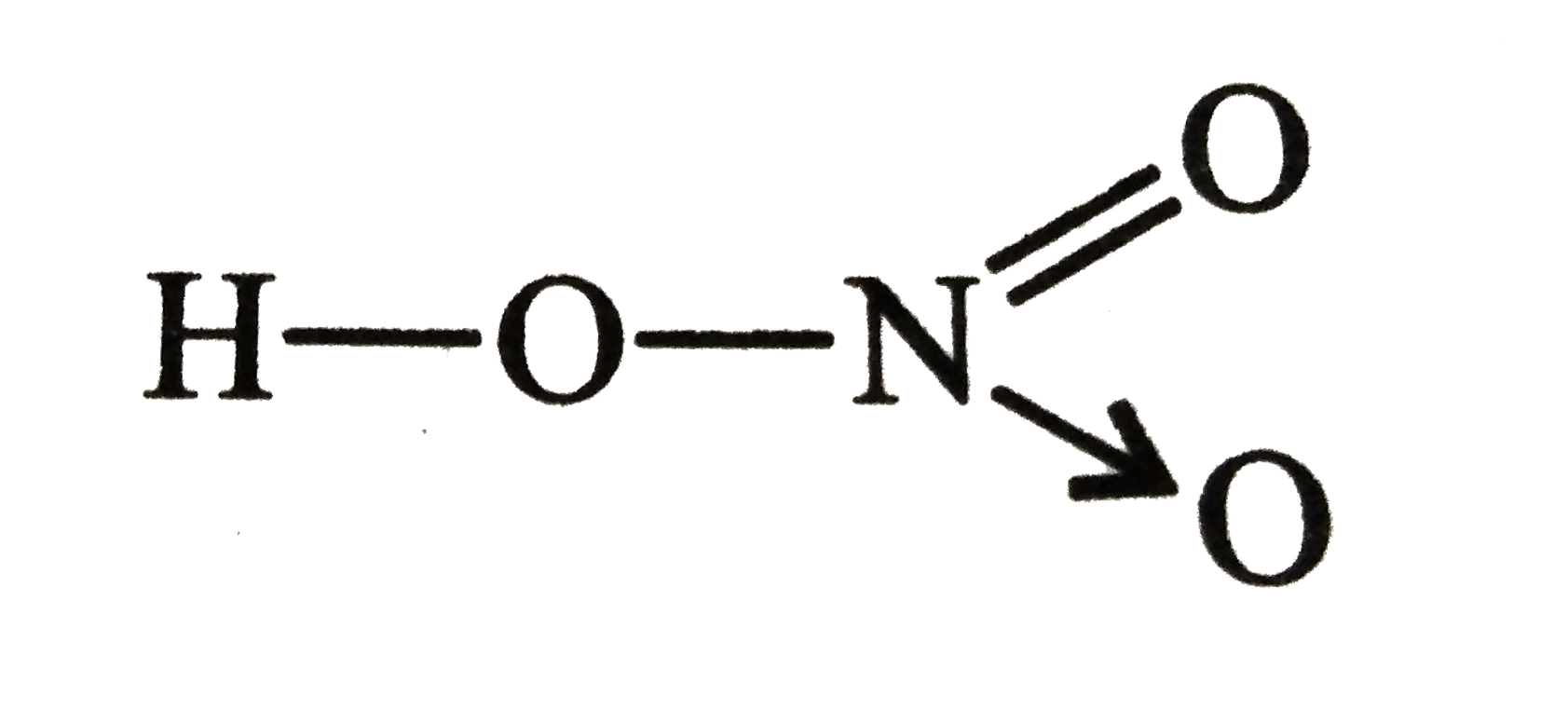
Thus, `H-C-=N`
Three bond `N` atom implies more electronegative
`1+a+3(-1)=0`
Oxidation number of `N=3(-1)= -3`
Oxidation number of `C, a= +2`
b. `Hul(N)C: H-C-=N`
Oxidation number of `H= +1`
Oxidation number of `N`
`{:("[=-2","+ (-1)" ,"+ 0]= -3"),({:("[For covalent"),("bond with C]"):},{:("[For covalent"),("bond with H"):},{:("[No contribution"),("for coordinate"),("bond]"):}):}`
According to fundamental concept `= -3`
`:. 1 + (-3) +a = 0 rArr a = +2`
c. `underline(N)O_(3)` : By rules , `1 + a + 3(-2) = 0 rArr a=+5`
By fundamental approach
`+N3`
Oxidation number of `H = +1`
Oxidation number of `N`
`{:("=+1","+ (+2)" ,"+ ( +2)=+5"),({:("[ covalent"),("bond with O]"):},{:("[Two covalent"),("bond with O,"),("N being less"),("electronegative"),("than O]"):},{:("[coordinate"),("bond]"):}):}`
d. `Kunderline(O_(2))`: A superoxide of `K`
Oxidation number of `K = +1`
Oxidation number of `O = a`
`1+2(a) = 0` and `a = -1//2`
e. `underline(Fe_(3))O_(4)`: `3(a) + 4(-2) = 0 rArr a = +8//3`
`Fe_(3)O_(4)` is a mixed oxide of `FeO.Fe_(2)O_(3)`.
Therefore, `Fe` has two oxidation numbers `+2` and `+3`, respectively. However, factually speaking, oxidation number in `Fe_(3)O_(4)` is an average of two values, i.e., `+2` and `+3`.
Therefore, average oxidation number `= (+2+(xx3))/(3) = +(8)/(3)`
`underline(I_(3))`: `1+3(a) = a rArr a = -1//3`
Since `KI_(3)` is `KI + I_(2)`, therefore, `I` has two oxidation numbers `-1` and `0`, respectively.
However, oxidation number of `I` and `KI_(3)` is an average of two values `-1` and `0`.
Therefore, average oxidation number
`= (-1 + 2(0))/(3) = -(1)/(3)`
g. `underline(N_(3))H`: `3(a) + 1 = 0 rArr a = -1//3`
h. `underline(Fe)(CO)_(5)`: Sum of oxidation number of `CO = 0`
`:. a+5(0) = 0 rArr a = 0`
i. `underline(Fe_(0.94))O`: `0.94(a) + (-2) = 0 rArr a = 200//94`
j. `underline(N)H_(2)underline(N)H_(2)`: Both `N` have same nature, therefore each `n` has oxidation number `-2`.
`underline(Fe)SO_(4)(NH_(4))_(2)SO_(4).6H_(2)O`:
Oxidation number of `Fe = a`
Sum of oxidation number for `(NH_(4))_(2)SO_(4) = 0`
Sum of oxidation number of `H_(2)O = 0`
Sum of oxidation number of `SO_(4)^(2-) = -2`
`:. a + (-2) + 0 + 6(0) = 0 rArr a = +2`
l. `underline(N)OCl`: `Cl-N=O` or use `NO^(o+)Cl^(Ө)`
Oxidation number of `N =- +1` (for covalent bonds with `Cl`)
Oxidation number of `N = +2` (for two covalent bonds with `O`)
Therefore, oxidation number of `N` in `NOCl = +3`
m. `NOunderline(Cl)O_(4)`: The compound may be written as `NO^(+)ClO_(4)^(Ө)` for `ClO_(4)^(Ө)`.
For `ClO_(4)^(Ө)`, let the oxidation number of `Cl = a`.
`:. a + 4(-2) = -1 rArr a = +7`
n. `Na_(2)[underline(Fe)(CN)_(5)NO]` : `NO` in ion complex has `H^(o+)` nature
`:. 2 xx 1 + [a + 5(-1) + (+1)] = 0`
`:. a = +2`
o. `[underline(Fe)(NO)(H_(2)O)_(5)]SO_(4)`:
`a + 1 + 5 xx 0 + (-2) = 0`
`rArr a = +1`
p. `Na_2S_(4)O_(6)`: `2(+1) + 4a + 6(-2) = 0`
`:. a = +5//2`
Here also, this value is the average oxidation number of `S`. The structure of `Na_(2)S_(4)O_(6)` is
`Na-O-underset(O)underset(darr)overset(O)overset(uarr)(S)-S-S-underset(O)underset(darr)overset(O)overset(uarr)(S)-O-Na`
Thus, oxidation nuber of each `S` atom forming coordiante bond is `+5`, whereas oxidation number of each `S` atom involved in pure covalent bonding is zero. Therefore, average oxidation number
`= (+5+5+0+0)/(4) = +(5)/(2)`
q. Dimethyl sulphoxide or `(CH_(3))_(2)underline(S)O`:
Oxidation number of `CH_(3) = +1`
Oxidation number of `O = -2`
`:. 2(+1) + a+(-2) = 0 rArr a = 0`
r. `Na_(2)underline(S_(2))O_(3)`: `2 xx 1 + 2 xx a + 3(-2) = 0 rArr a = +2`
Here too, it is the average oxidation number.
The sturcture of `Na_(2)S_(2)O_(3)` is
`Na-S-underset(O)underset(darr)overset(O)overset(uarr)(S)-O-Na`
The oxidation number of `S` involved in coordinate bond, i.e., donor `S` atom, is `+5`. The oxidation number of other `S` atom is `-1`.
s. `Caunderline(Cl_(2))`: In bleaching powder, two `Cl` atoms are as `Ca(Cl)Cl`, i.e., one as `Cl^(Ө)` having oxidation number `-1` and other as `OCl^(Ө)` having oxidation number `+1`.