Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write a balanced equaiton when copper reacts with nitric acid, a brown...
Text Solution
|
- Write a balanced equaiton when copper reacts with nitric acid, a brown...
Text Solution
|
- Copper reacts with conc. Nitric acid. Give balanced reaction.
Text Solution
|
- Write the balanced chemical equation for the reaction. When copper rea...
Text Solution
|
- Name the gas formed when copper reacts with dilute nitric acid
Text Solution
|
- Hot and conc. Nitric acid when reacts with copper, the gas obtained is
Text Solution
|
- An aqueous solution of a gas (X) -turns red litmus blue. When excess o...
Text Solution
|
- An aqueous solution of a gas (X) turns red litmus blue. When added in ...
Text Solution
|
- Copper reacts with nitric acid . A brown gas is formed and the solutio...
Text Solution
|
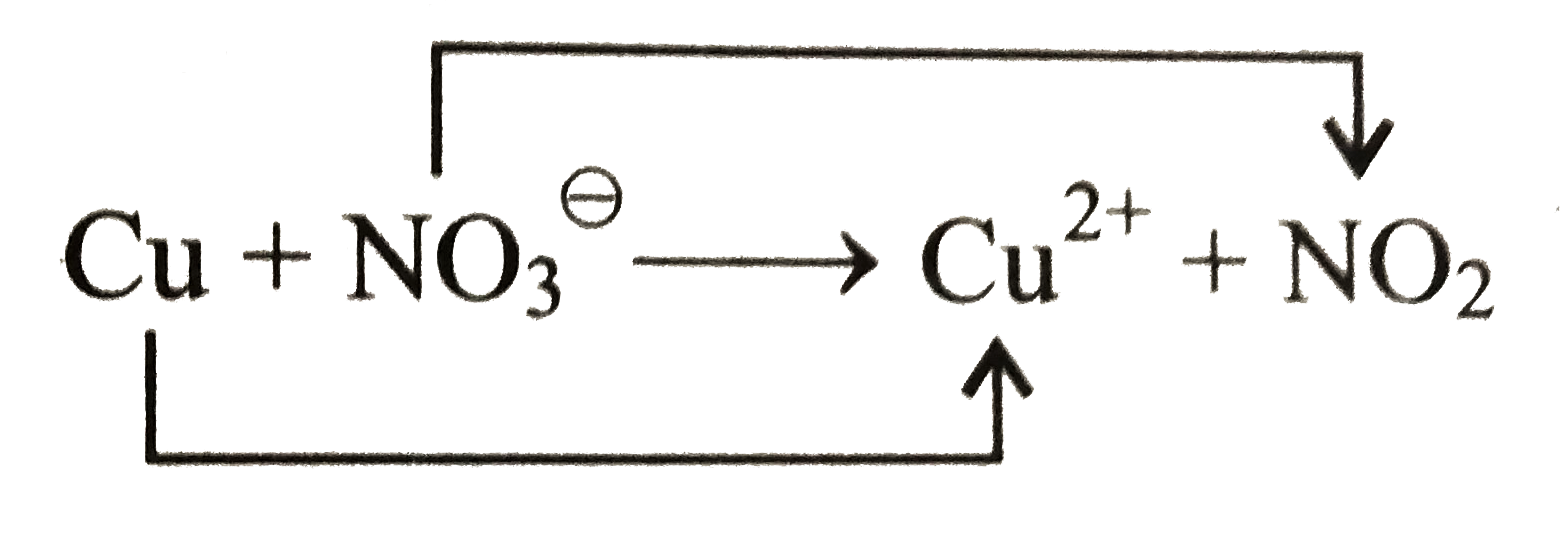
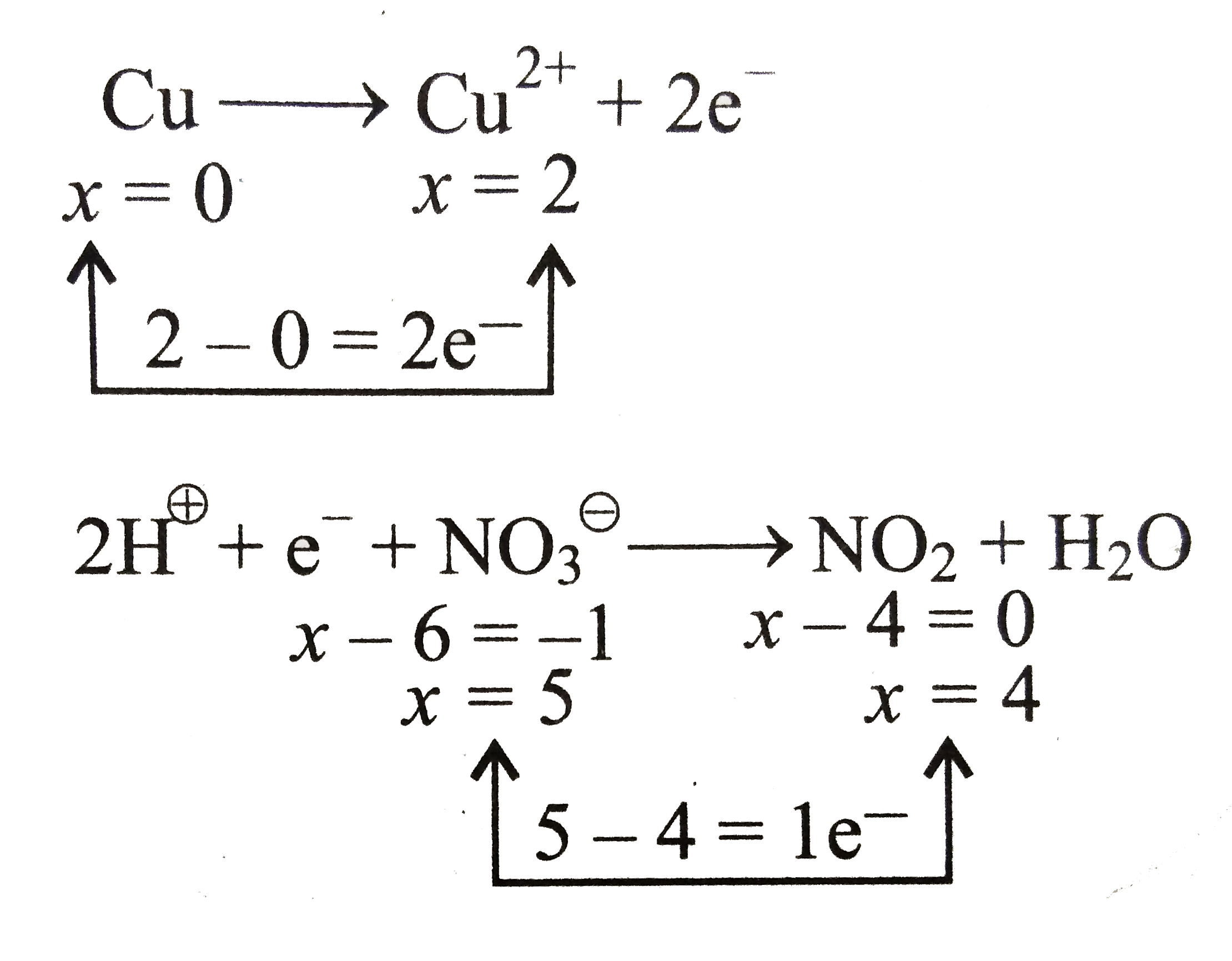 ….(i)
….(i)