Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
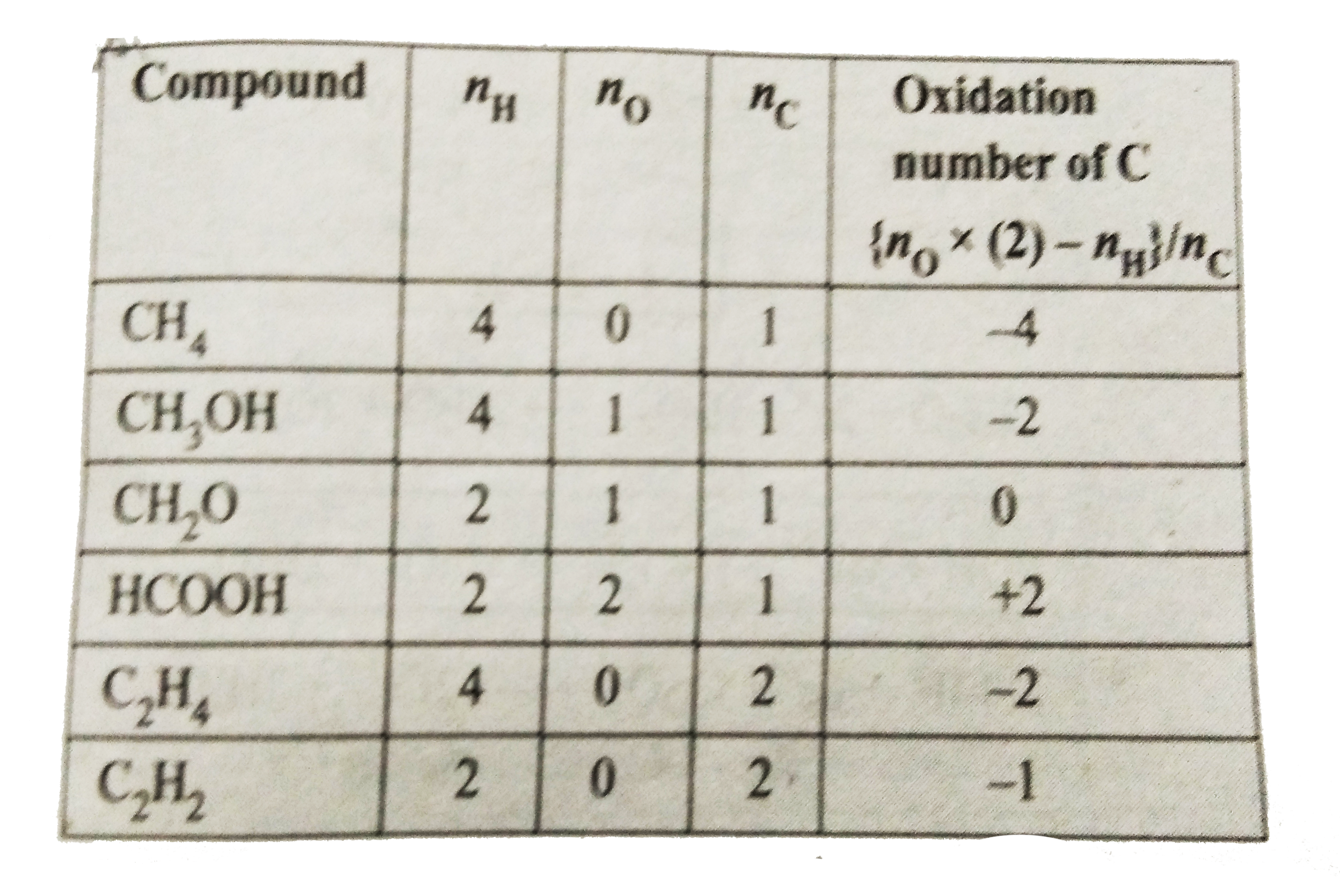
- Find the oxidation number of carbon in the following compounds: CH(3)O...
Text Solution
|
- Find the oxidation number of carbon in the following compounds: CH(3)O...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- Amongest the following maximum number of compounds which give iodofor...
Text Solution
|
- निम्नलिहित यौगिकों को कवथनांकों के बढ़ते कर्म में व्यवस्थित कीजिए - C...
Text Solution
|
- निम्नलिखित यौगिकों में प्रत्येक कार्बन की संकरण अवस्था बताइए- CH(2)=...
Text Solution
|
- Arrange the following alcohols in the order of increasing number of ca...
Text Solution
|
- Identify the type of the following reaction of carbon compounds CH(3)-...
Text Solution
|
- Identify the type of the following reaction of carbon compounds CH(3)-...
Text Solution
|
 .
.