Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
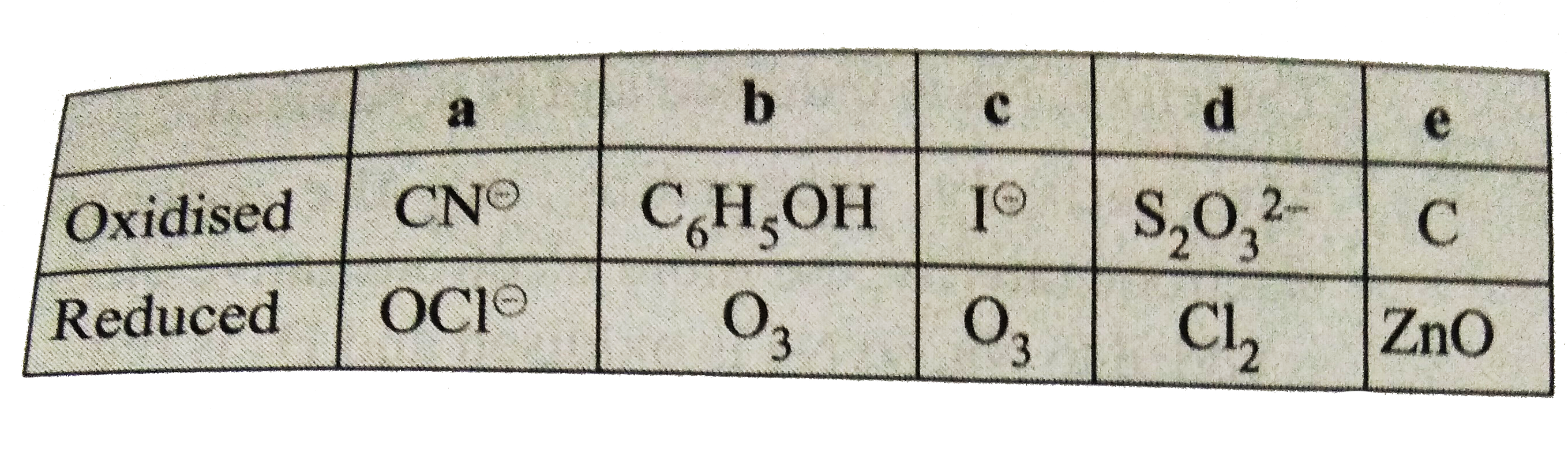
- Indicate the species which are oxidised and reduced in the following r...
Text Solution
|
- Classify the following redox reactions: a. N(2)(g)+O(2)(g)rarr 2NO(g) ...
Text Solution
|
- Balance the following equations: a. BaCrO(4) + KI+HClrarrBaCl(2)+I(2...
Text Solution
|
- Indicate the species which are oxidised and reduced in the following r...
Text Solution
|
- Balance the following chemical reactions (by ion electron method) (a...
Text Solution
|
- Write balanced net ionic equations for the following reactions in basi...
Text Solution
|
- Balanced the following equations: (a) H(2)O(2)+H^(o+)+Fe^(2+)rarr H(2)...
Text Solution
|
- Write correctly balanced half reactions and overall equations for the ...
Text Solution
|
- Balance the following equations by the ion electron method: a. MnO(4...
Text Solution
|