A
B
C
D
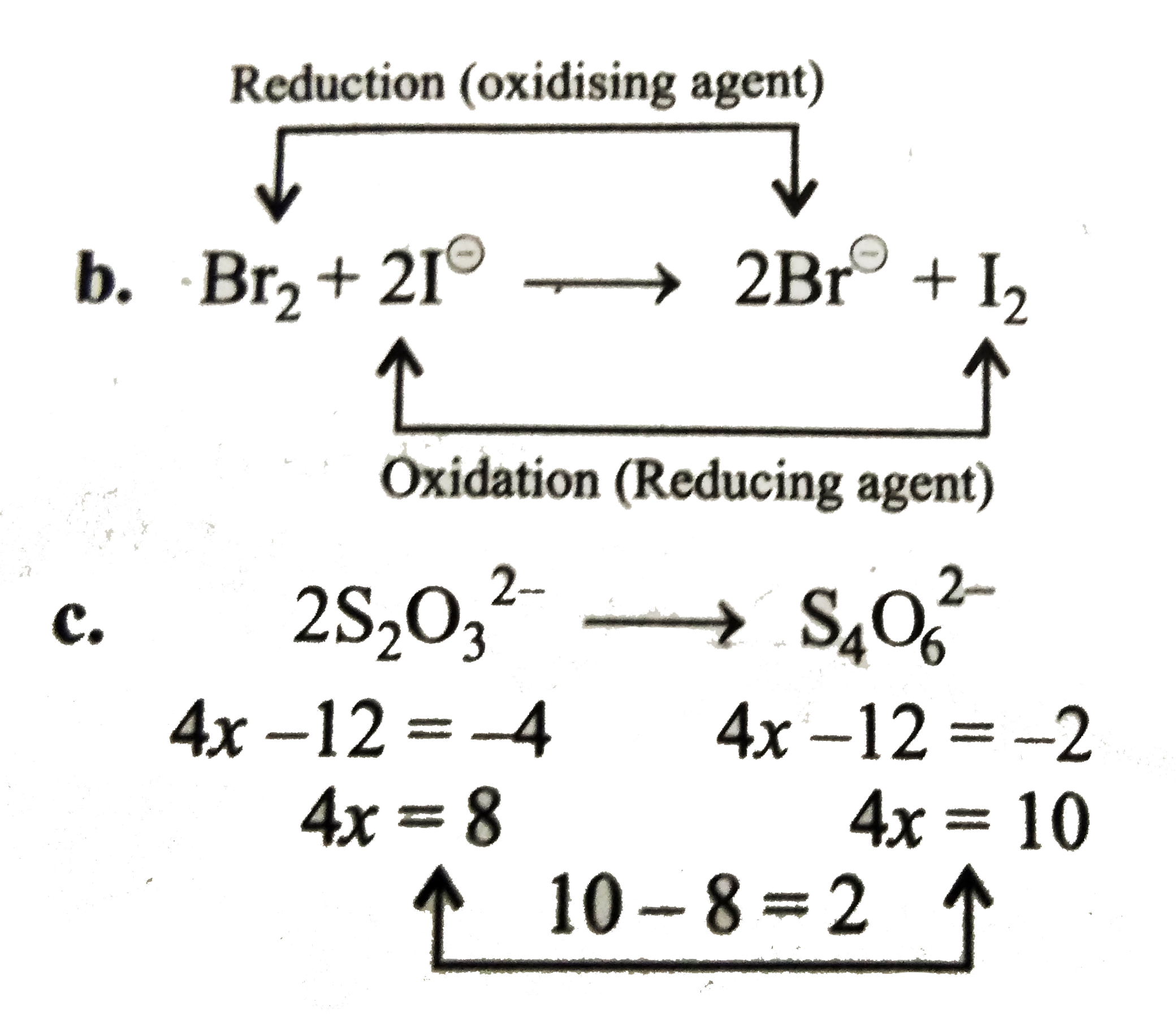
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation and reduction process involves the transaction of electrons....
Text Solution
|
- Oxidation and reduction process involves the transaction of electrons....
Text Solution
|
- Oxidation and reduction process involves the transaction of electrons....
Text Solution
|