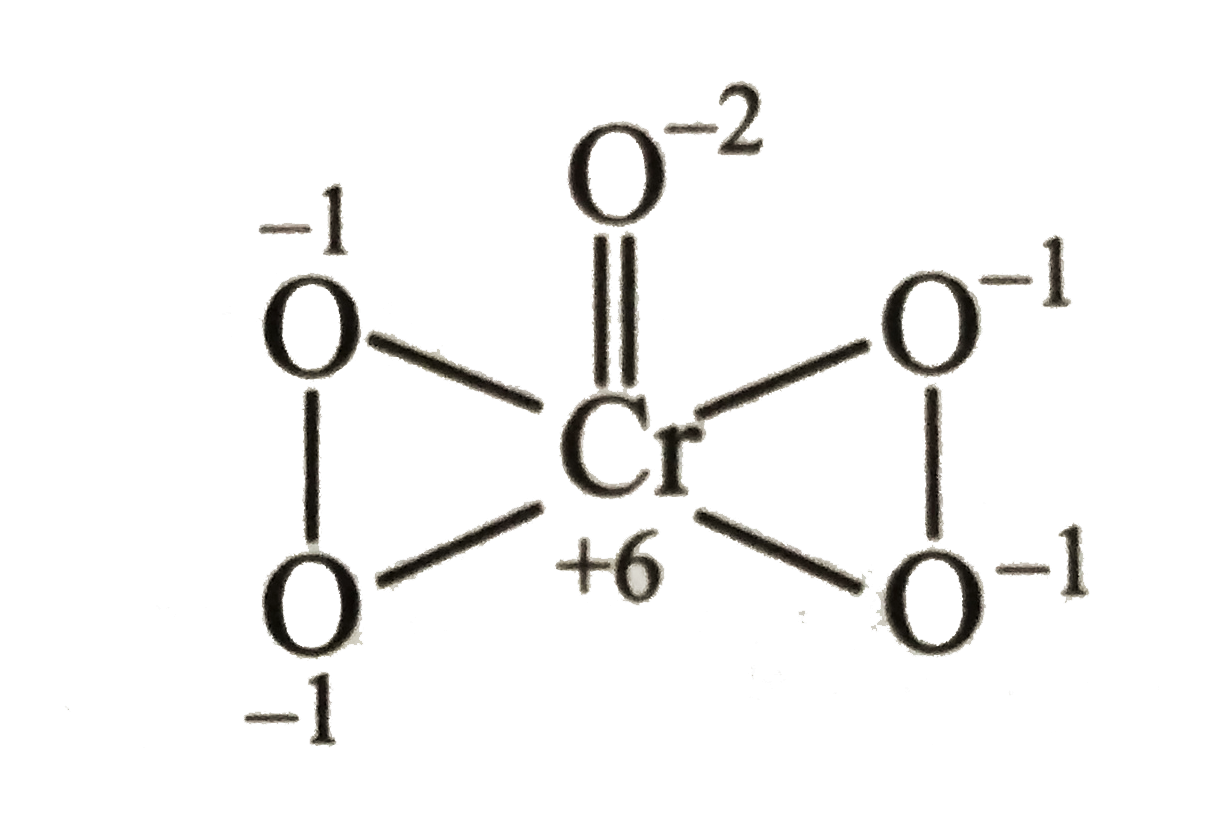
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The oxidation number of Cr is +6 in
Text Solution
|
- The oxidation number of Cr is +6 in
Text Solution
|
- The oxidation number of Cr in [Cr(C(6)H(2))(2] is 0 (b) +2 (c ) +3...
Text Solution
|
- The oxidation number of Cr is +6 in :
Text Solution
|
- The oxidation number of Cr is +6 in:
Text Solution
|
- The oxidation number of Cr in Cr(CO(6)) is :
Text Solution
|
- In which of the following compounds of Cr, the oxidation number Cr is ...
Text Solution
|
- Assertion :- Oxidation number of Cr in Cr(CO)(6) is zero. Reason :- Cr...
Text Solution
|
- The oxidation number of Cr in [Cr(NH(3))(6)]Cl(3) is
Text Solution
|