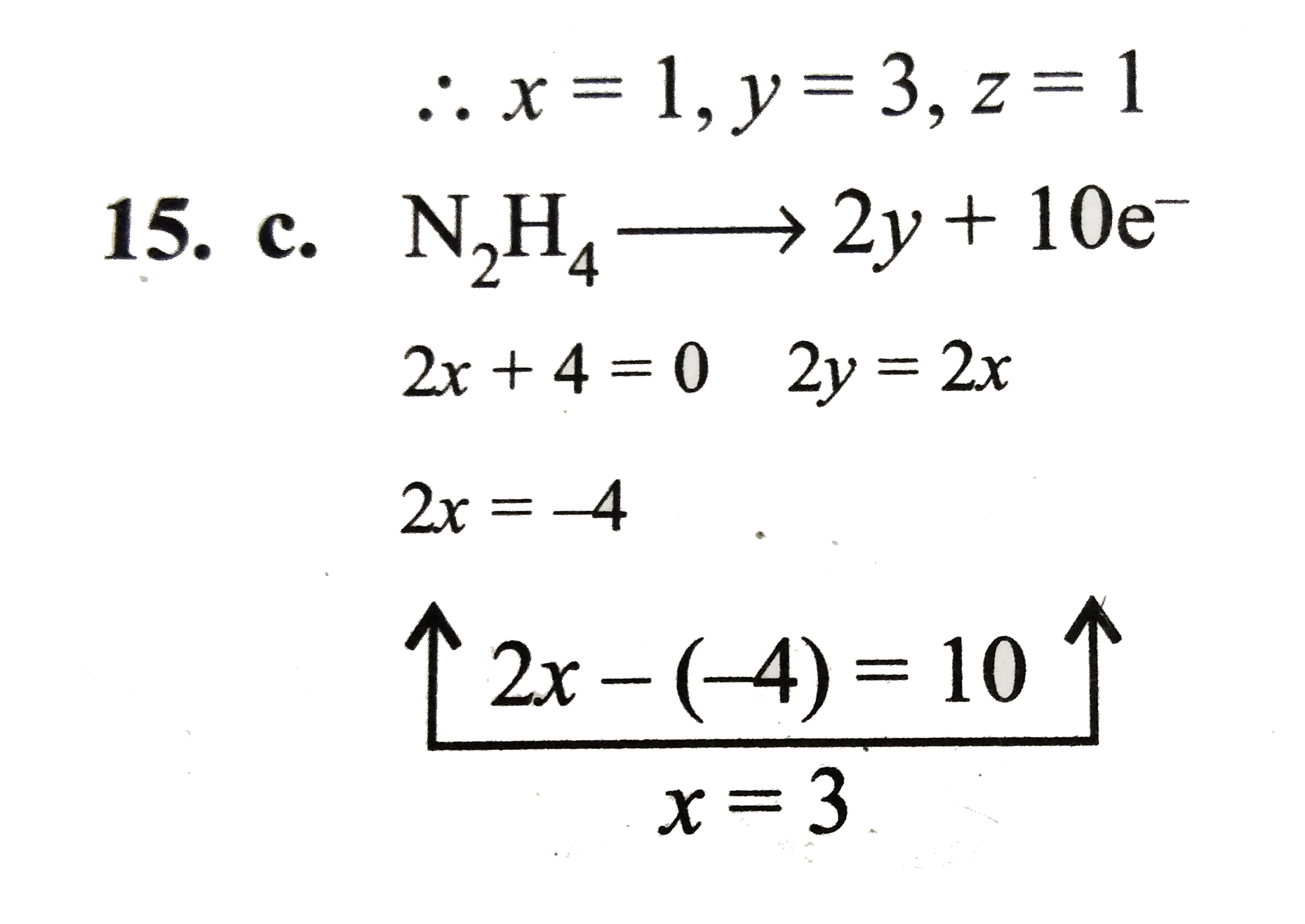A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses 10 moles of electrons of form a new compoun...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses 10 moles of electrons to form a new compoun...
Text Solution
|
- One mole of N(2)H(4)¸ loses 10 moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|