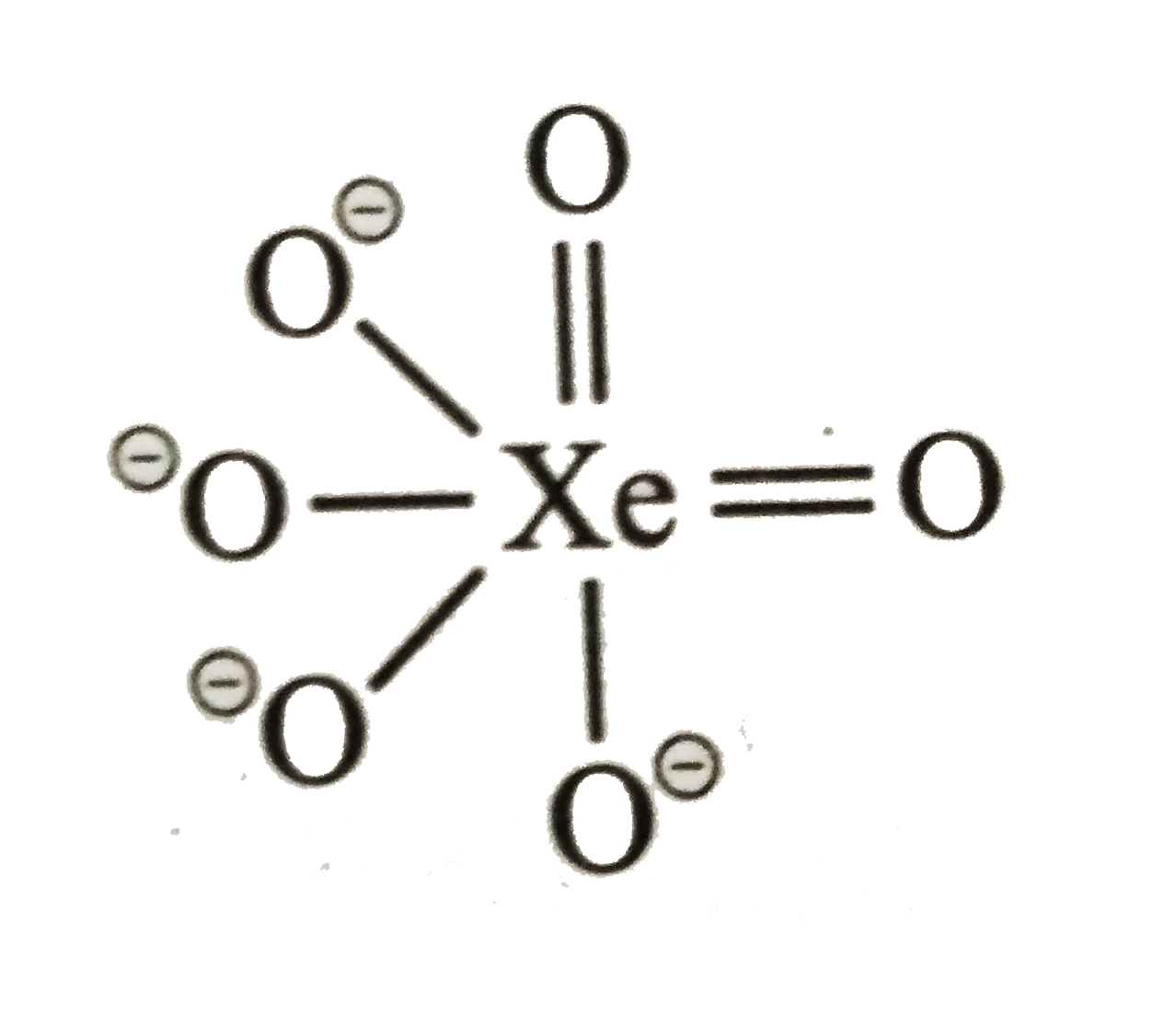A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of peroxide bonds in perxenate ion [XeO(6)]^(4-) is
Text Solution
|
- The number of peroxide bonds in perxenate ion [XeO(6)]^(4-) is
Text Solution
|
- Assertion (A): Sodium perxenate (Na(4)XeO(6)) reacts with NaF in acidi...
Text Solution
|
- The xenate ion (HXeO(4)^(-)) undergoes disproportionation reaction to ...
Text Solution
|
- The number of P pi- d pi 'Pi' bonds present in XeO(3) and XeO(4) molec...
Text Solution
|
- The number of pi bonds in perxenate ion is :
Text Solution
|
- Perxenate ion is
Text Solution
|
- Find the number of compounds of Xe which is/are associated with 180^(@...
Text Solution
|
- The correct statement regarding perxenate ion (XeO(6)^(4-)) is:
Text Solution
|