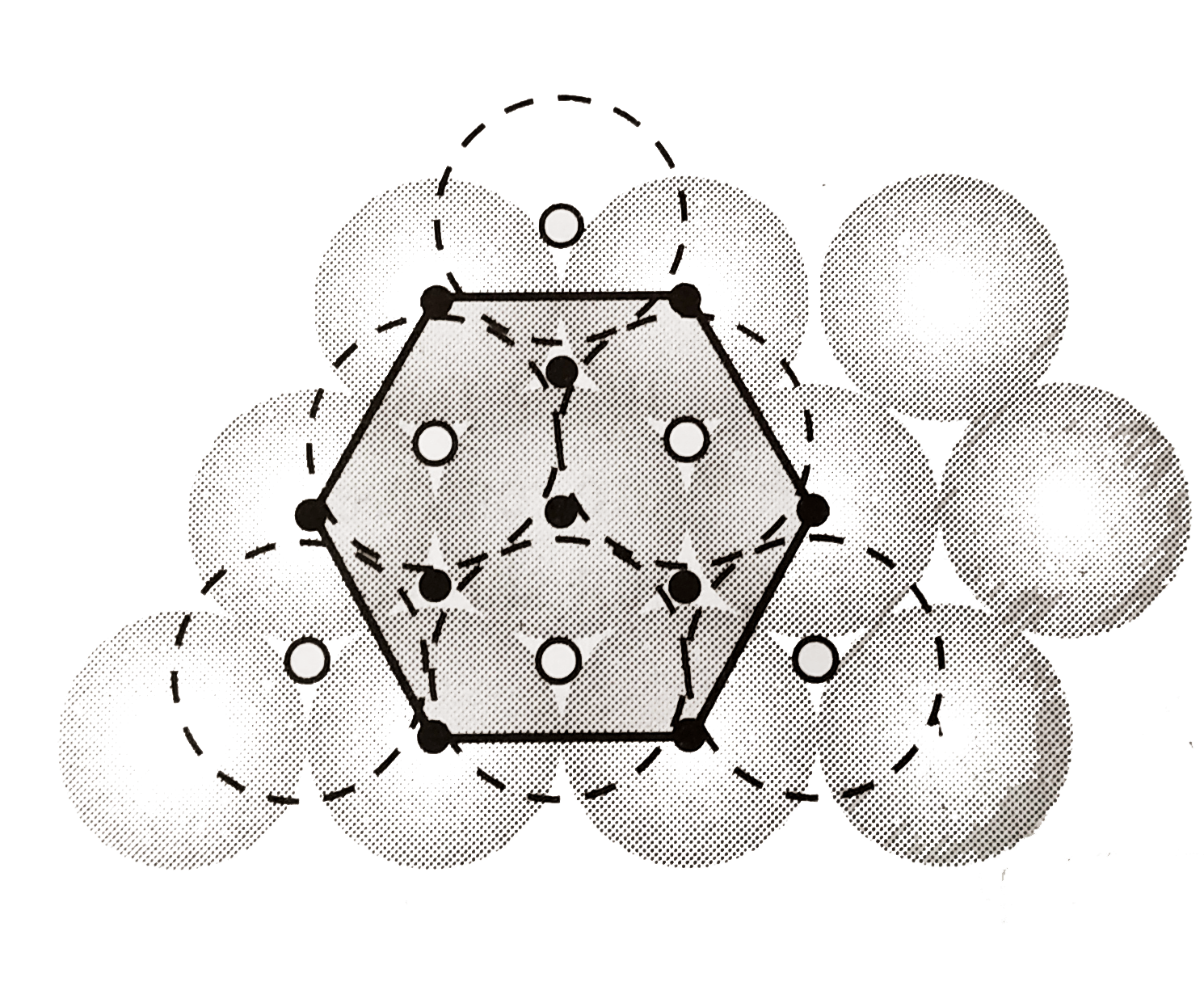
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In hexagonal close packing of spherer in three dimensions.
Text Solution
|
- In hexagonal close packing of shpere in three dimesions, which of the ...
Text Solution
|
- Mathc the type of packing given in Column I with the itmes given in Co...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Select the correct statement(s) related to hexagonal close packing of ...
Text Solution
|
- Between square and hexagonal close packing in two dimensions, which on...
Text Solution
|
- Hexagonal closely packed| Cubicaly closely packed
Text Solution
|