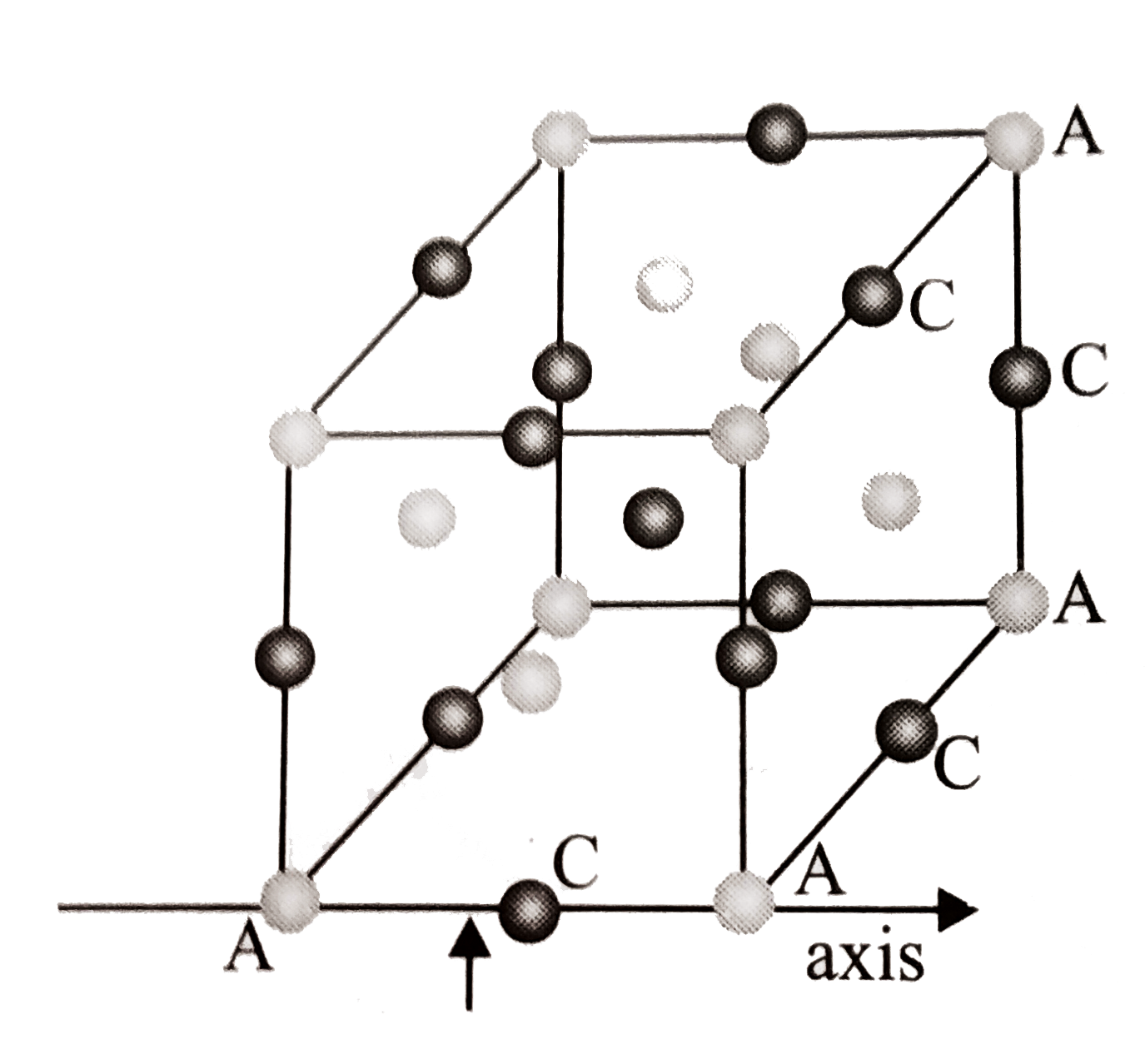
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A compound made of particles A, B, and C forms ccp lattice. Ions A are...
Text Solution
|
- A forms ccp lattice B occupy half of the octahedral voids and O occupy...
Text Solution
|
- In a compound, oxide ions are arranged in ccp. One-sixth of the tetrah...
Text Solution
|
- A compound made of particles A, B, and C forms ccp lattice. In the lat...
Text Solution
|
- A compound made of particles A and B. A forms fc c packing and B occup...
Text Solution
|
- In a crystal of an ionic compound, the anions B form a close packed la...
Text Solution
|
- In a crystal of an ionic compound, the ions B form the close packed la...
Text Solution
|
- In a crystal of an ionic compound, the ions B form the close packed la...
Text Solution
|
- In a compound, atoms A occupy 3//4 of the tetrahedral voids and atoms ...
Text Solution
|