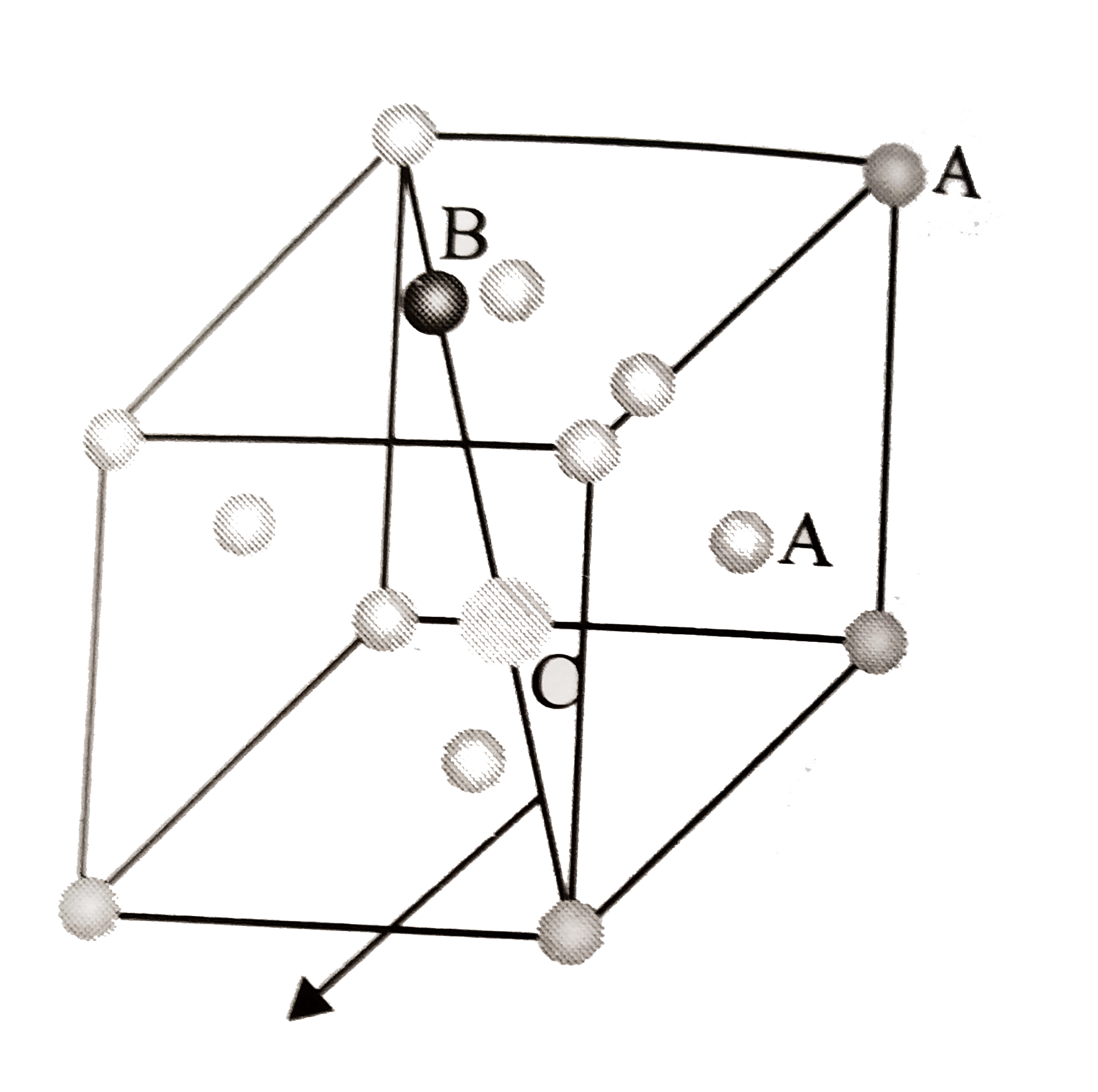
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A compound made of particles A, B, and C forms ccp lattice. In the lat...
Text Solution
|
- A compound made of particles A, B, and C forms ccp lattice. Ions A are...
Text Solution
|
- In a crystal of an ionic compound, the anions B form a close packed la...
Text Solution
|
- In a crystal of an ionic compound, the ions B form the close packed la...
Text Solution
|
- A body centred cubic lattice is composed of anions Q and cations P, wh...
Text Solution
|
- In a crystal of an ionic compound, the ions B form the close packed la...
Text Solution
|
- In a compound, atoms A occupy 3//4 of the tetrahedral voids and atoms ...
Text Solution
|
- A binary solid A^(+) B^(-) has a structure with B^(-) ions constitutin...
Text Solution
|
- A(2)B has antifluorite structure (B forms FCC lattice and A occupies t...
Text Solution
|