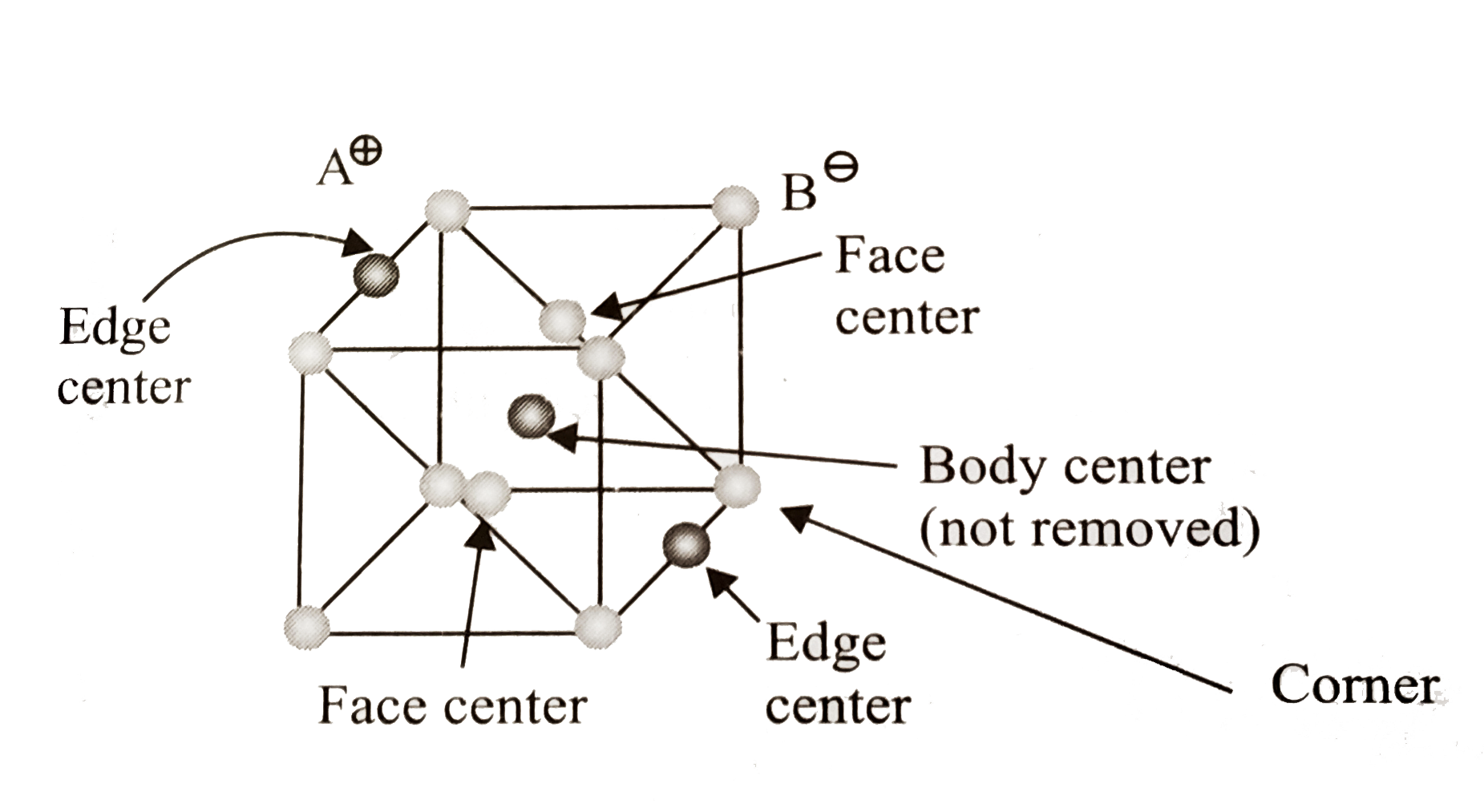
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a solid having rock salt structure, if all the atoms touching one b...
Text Solution
|
- In a unit cell, atoms (A) are present at all corner lattices, (B) are ...
Text Solution
|
- In a unit cell, atoms (A) are present at all corner lattices, (B) are ...
Text Solution
|
- In a solid (AB) having rock salt type structure all the atoms along bo...
Text Solution
|
- Atoms A,B,C, D are present at corners , face-centres , body-centres an...
Text Solution
|
- A solid with a formula AB has the rock-salt type of structure. If all ...
Text Solution
|
- Crystalline solid AB has face-centred cubic unit cell. Corners and the...
Text Solution
|
- An AB solid has CsCl type of structure, where A occupies corner. If th...
Text Solution
|
- In a solid 'AB' having rock salt arrangement ( NaCl structure), 'A' at...
Text Solution
|